- Fabrication and characterization of a novel foam ceramic material based on coal gasification slags
Hudie Yuan, Hongfeng Yin*, Yun Tang, Yalou Xin, Xilou Pu and Hao Zhang
Provincial Experimental Teaching Demonstration Center for Functional Materials, College of Materials Science & Engineering, Xi’an University of Architecture & Technology, 710055 Xi’an, China
In this paper, the feasibility
of recycling coal gasification slag by high temperature foaming process to
fabricate foal ceramic insulation board has been investigated. Silicon carbide
(SiC) was added as the foam agent, and the foaming mechanism was also
discussed. The results showed that utilization rate of the coal gasification
prepared to the foam ceramic new wall material was up to 77% and the best
dosage of SiC was 3%. Moreover, the relatively optimum parameters were
obtained at the sintering temperature of 1,160 ºC for 3h with 77% slags, 20% clay and 3% SiC.
Furthermore, compression strength reaches 1.18 MPa, the bulk density and heat conductivity
coefficient value are 0.21 g/cm3 and 0.05 W/(m·k), respectively.
Keywords: Foam ceramic, Walling material, Coal gasification slag; SiC, Heat conductivity coefficient
Recently, with the implement of related policies such as
“about speeding up the enforcement advice to promote the development of green
building in China”, “green building build action plan” and the new “code for
fire protection design of buildings”, and further progress of building energy
conservation [1-3]. The requirement of thermal insulation material for exterior
wall of civil building is significantly increased.
Especially, foam ceramic insulation board with
its many advantages gradually attracted attention. Foaming ceramic is a
kind of ceramic material with mine tailings and industrial solid waste as the
main raw materials, adds special foaming agent, and forms amount of uniform
closed pores by sintering at high temperature, which are different from the
honeycomb ceramics using the open porosity technique [4-6]. As a new material,
the foam ceramic possesses excellent comprehensive properties such as
light-weight, high-strength, thermal insulation, fire retardant, sound
insulation, and the characteristics of quick and clean construction, low
building load and recyclability, etc [7-10]. Precisely, its production
process can consume kinds of industrial solid waste, which is
conducive to environmental protection, the foam ceramic become a new kind of green
environmental protection building material in the field of new wall material
and interior and exterior decoration [11-13].
The industrial solid waste can be used as the main raw
material source of the foaming ceramics, which is also an
important factor for the sustainable development of the
foaming ceramics industry. As one kind of solid wastes, coal
gasification slag is an inevitable by-product from entrained-flow coal
gasification [14], which is comprised of coal ash, fluxing agent and residual
carbon. The improper disposal of coal gasification slag has resulted in
environmental pollution. According to the literature, the
studies on coal gasification slag mainly focused on three aspects: (1) general
characterization of the slags, such as composition, morphology,
physico-chemical characteristics of residual
carbon, characteristics and catalytic actions of inorganic
constituents, particle size distribution and thermal expansion property
[15-18]. In addition, in order to improve the efficiency of coal gasification,
residual carbon in the slag was investigated extensively
[19, 20]; (2) rheological behavior and flow
properties of the slags, which determine the slag tapping temperature in the
gasifier [21-25]; (3) the utilization of the slag. Accordingly,
the environmentally safe utilization of the waste slags must be
addressed and developed. Recently, most of the slags were conducted in cement
(39.3%), landfill (33.4%), road and flyover (5.0%), agriculture (2.9%), bricks
and tiles manufacture (12.3%) [26], in addition, the SiAlON ceramics prepared
by the slags were investigated by our research group [27].
Thus far, many different types of industrial wastes are
used as the raw materials of foam ceramic, such as fly ash, blast furnace slag,
porcelain waste etc [28-30], but there are few studies about foam ceramic based
on coal gasification slags. In this work, efforts had been made to prepare foam
ceramic wall material. The coal gasification slag from Xianyang chemical
industry Co. LTD was used as raw material and SiC was used as foaming agent.
Furthermore, the high-temperature foaming process was employed. Besides, the
foaming mechanism was discussed. The significance of this work lies in two
aspects: First, utilization rate of the coal gasification prepared to the foam
ceramic new wall material was up to 77%. As we know, coal gasification slag is
an inevitable by-product from entrained-flow coal gasification, which has
resulted in environmental pollution. In this paper, comprehensive utilization
rate of coal gasification slag was significantly improved,
which would be alleviated the environmental pollution
caused by coal chemical industry; Second, the aim products in this study
possess the performance such as lightweight, thermal
insulation and fireproofing, which is the green product that
building materials market needs today. In other words, this work not only can
verify the feasibility of this foam ceramic as a new inorganic building
insulation material, but also provide an important technical support for the comprehensive
utilization and industrialization of foam ceramic insulation board from coal
gasification slag. Moreover, it provides new ideas for the greenization of new
wall materials.
Characterization
of raw materials
In this work, the primary raw material was coal
gasification slag (termed as CGS), which was provided by Xianyang chemical
industry co. LTD (Xianyang, Shaanxi, China). And the clay came from the local
resource. Moreover, the raw materials were dried in an oven at 110 ºC for 24 h.
And for the materials, X-ray fluorescence (XRF) was employed to detect the
chemical composition of raw materials, and X-ray diffraction (XRD, using a
D/Max 2550V, Akashima, Japan) was used to investigate the crystalline phases of
them.
The results of XRF and XRD were shown in Table 1 and Fig.
1, respectively. It can be seen CGS mainly contains 38.39 wt.% SiO2,
31.13 wt.% C and 14.66 wt.% Al2O3,
with a small quantity of Fe2O3(3.39 wt.%),
CaO (5.85 wt.%), K2O (1.64 wt.%), MgO (1.02 wt.%), Na2O
(0.89 wt.%). Furthermore, high content of SiO2 (68.56 wt.%), Al2O3
(17.66 wt.%) and Fe2O3(4.64 wt.%) contained in clay
that locally produce. Besides, it is observed from Fig. 1, in CGS,
the major crystalline phase is Quartz (SiO2),
while in clay, except for the Quartz (SiO2), the Na0.3(Al,Mg)2Si4O10(OH)28H2O
and KAl2Si3AlO10(OH)2 present as
well.
Preparation
of samples
In this paper, the main raw material was the CGS,
meanwhile, the clay was added in appropriate content, and small quantity of SiC
(3 wt.%) was added as foaming agent. And the proportion of raw materials was
shown in Table 2. In order to mix raw materials well together, all raw
materials were milled in de-ioned water using Si3N4
milling media in a planetary ball mill at a constant speed of 320 rpm for 6 h.
The mixed slurry was dried at 110 ºC in oven for 24 h, and sieved through
a 200-mesh screen to remove agglomerations. Then, the
dried materials were made into round particles by spray
granulation on a disc granulator, and the particles formed by the granulator
were placed in an oven to be dry at a gradient temperature (30 ºC, 50 ºC and 80
ºC for 2 h, 110 ºC for 12 h) in order to prevent the particle from cracking.
After drying, three particle size distributions (1.4~2.36 mm, 1~1.4 mm and
0.25~1 mm) were obtained by passing through a sieve of 8 mesh, 12
mesh, 16 mesh and 60 mesh, respectively. Then,
samples were prepared directly by heating the particles in a furnace at a
various final temperature in the range of (1,140~1,160 ºC at an interval of 10
ºC). The heating rate was 5 ºC/min, and then the sintered samples were cooled
naturally.
Characterization
of sintered samples
For the mixed powder, in order to determine the weight
losses and the thermal behavior of high temperature,
the thermal gravimetric-differential scanning calorimeter
analysis (TG-DSC, STA449F3, Selb, Germany) was studied. The test condition of
the temperature range was from the 20 to 1,400 ºC with a heating rate of
15 ºC/min at air atmosphere.
For the sintered samples, volume density, compressive
strength, thermal conductivity, phase composition and microstructure were
characterized systematically. First, the volume density of the sintered
samples, according to GB/T5486-2008 test method for inorganic hard adiabatic
products, was evaluated [31]. In this test, the samples were dried at 110 ºC
for 12h, and then restored to room temperature, then measured the weight
(termed as M); The geometry of the sample were measured and its volume (termed
as V) was calculated. Finally, the volume density (ρ) was calculated by using
the following formula (1)

Second, compressive strength was tested by compression testing
machine (Yes-600, Jinan, China), according to GB/T5486-2008
test method for inorganic hard adiabatic products as well. Third, thermal
conductivity was detected by Thermal Conductivity Measuring Instrument (TC3000,
Xi’an, China); Forth, Phase identification of the samples was performed via X‑ray
diffractometry (XRD, using a D/Max 2,550 V, Akashima,
Japan) applying CuKα1 (λ = 1.5405981 Å)
radiation; And then the morphology of the samples was investigated
by scanning electron microscopy (SEM, model Auriga, Zeiss, Oberkochen,
Ostalbkreis, Germany).
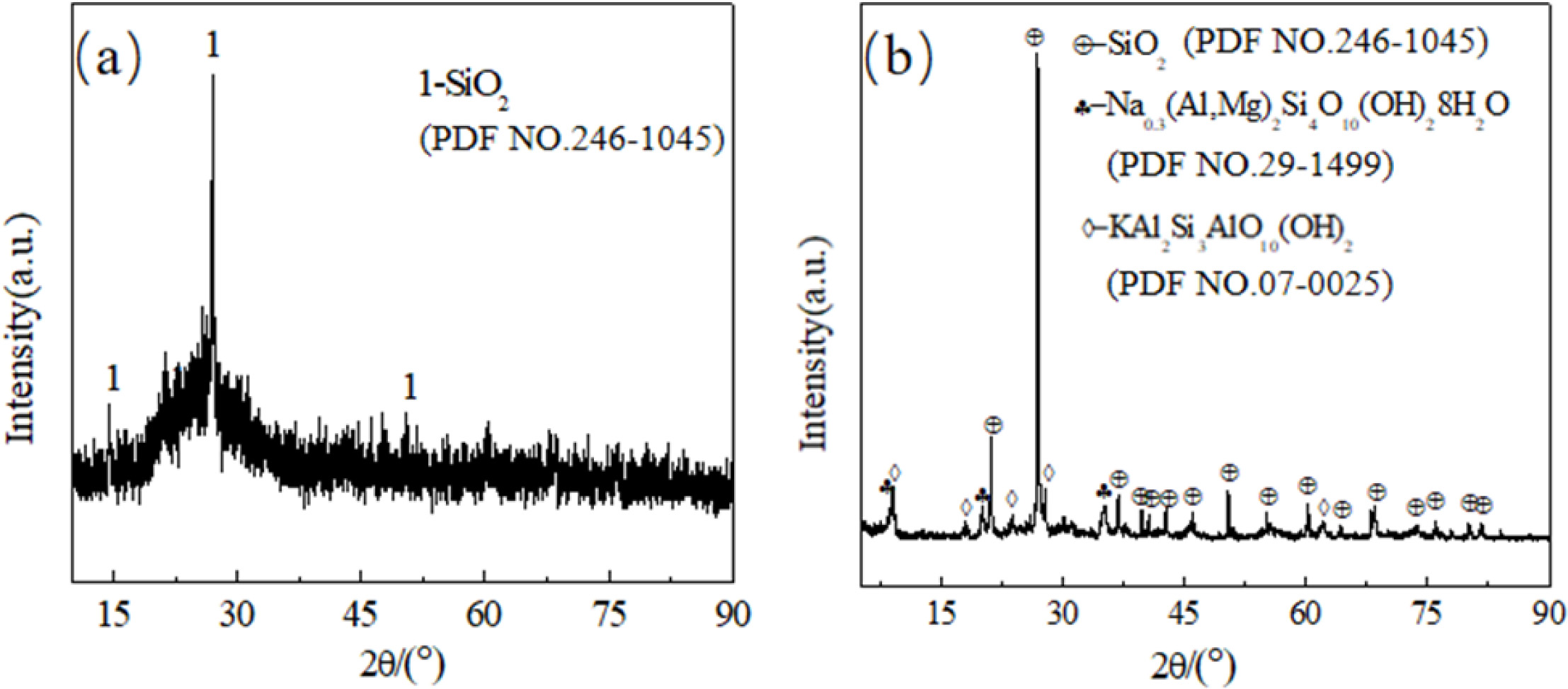
|
Fig. 1 X-ray diffraction of CGS (a) and clay (b). |
The
foaming mechanism of SiC in CGS
Considering the foaming temperature of SiC is varied from
900 ºC to 1,300 ºC; Moreover, according to the previous study [30] of our
research group, the lowest eutectic point of each component of CGS is 1,170 ºC.
For this reason, SiC was used as the foaming agent.
For better research on the foaming mechanism of SiC in
CGS, the combustion thermodynamics and kinetics for SiC were studied. The Gibbs
free energy of some reactions of SiC with oxygen at 25 ºC and
1,170 ºC were calculated by FactSage 7.1 (Reaction module, Beijing,
China). As displayed in Table 3, It can be obviously
seen that all the Gibbs freeenergy was negative number.
Therefore, based on the thermodynamics, SiC was unstable both at room temperature
and the melting temperature of CGS system. And it can be reacted with oxygen
and caused gas released. From a kinatics point of view, the combustion reaction
of SiC was not smoothly. Numerous studies indicated that there existed a dense
SiO2 envelope layer that muffled SiC during the reaction
[32, 33]. In that case, the rate of oxidation reaction
between SiC and O2 was controlled by diffusion,
and what’s more, the diffusion rate of oxygen in the SiO2
envelope layer was only 10–14~10–15 cm2/s.
Hence, SiC (inside of the raw material system) reacted with the
oxygen was effectively stopped. Moreover, the TG-DSC of the
raw material system from room temperature to
1,400 ºC in air atmosphere had been tested for study the reaction
temperature of the raw material system and the foaming mechanism of SiC in CGS.
The result was shown in Fig. 2. Intuitively, there exists a weight loss of
5.36% during the testing process, and this weight loss may be the impact of
impurities and oxidation of residual carbon. In addition, in Fig. 2 there is an
endothermic peak from 950 to 1,230 ºC, which
indicates the generation of liquid phase and oxidation of SiC at
this temperature range [34-36]. According to the research of Hao Wang [30], the
TG-DSC of SiC from room temperature to 1,380 ºC in air atmosphere showed
that the weight of the system increased; compared with the
theoretical calculation, the weight increment could reach 50%
when the SiC fully reacted with O2. Nevertheless, seen from Fig.
2, there was no noticeable increase in weight for CGS system. This might be the
result of the low content of SiC and higher content of residual
carbon. As to the temperature of the endothermic peak, the
endothermic peak of pure silicon carbide appeared at about 1,110 ºC, while
the CGS system appeared at about 1,198 ºC, which could explain as follows:
the simulated ternary phase diagram of the raw material system showed that the
lowest eutectic point of the system was 1,170 ºC, and with the temperature
increasing, the liquid phase quantity persistently increased, which would
destroy the SiO2 protective layer, in that case, the temperature was
up to about 1,198 ºC, the SiC inside can reacted with oxygen sufficiently.
Hence, As displayed in Fig. 3, the preparation of foaming
ceramics by the CGS system can be expressed as: Firstly, owing to contain
certain SiO2 in the system and generate the SiO2
protective layer covered on the SiC, SiC showed relatively stable at lower
temperature; Secondly, the combustion of residual carbon some extent provided
the impetus for the reaction of SiC with oxygen until the residual
carbon reacted completely; Thirdly, the liquid phase generated
with the sintering temperature increased, which destroyed the SiO2
protective layer and gave rise to the increase of O2 diffusion rate,
meanwhile, a capillary pressure formed by high temperature liquid phase could
make sample densified and fix the gas in the liquid phase as well. In this case,
the closed pores were formed in samples.
The
effects of the grain size and sintering temperature on samples
In this paper, the process of spray granulation was employed.
For sample 1 to 3, heating at the temperature (1,140-1,160 ºC),
the effects of the grain size on samples and sintering temperature
were shown in Fig. 4. Seen from Fig. 4(a), as the particle size and sintering
temperature increased, the density of the samples increased. As shown in Fig.
4(b), with the increasing of the particle size, the compress strength of the
samples increased, While, the compress strength of the samples decreased. This
phenomenon can be explained that, the particle size is negatively correlated
with the specific surface area. The smaller the particle size, the larger the
specific surface area, the more fully in contact with oxygen, the
higher the heat transfer efficiency, the more liquid phase can be produced at
the same temperature, and the more fully reactive between SiC and O2.
In that case, the content of the pore increased. For this reason, the density
of samples decreased. Meanwhile, at higher sintering temperature, temperature
becomes the most important factor, so the compress strength of the
samples was lowest at 1,160 ºC. Whereas, the
strength still meets the standard requirements for
lightweight wall materials.
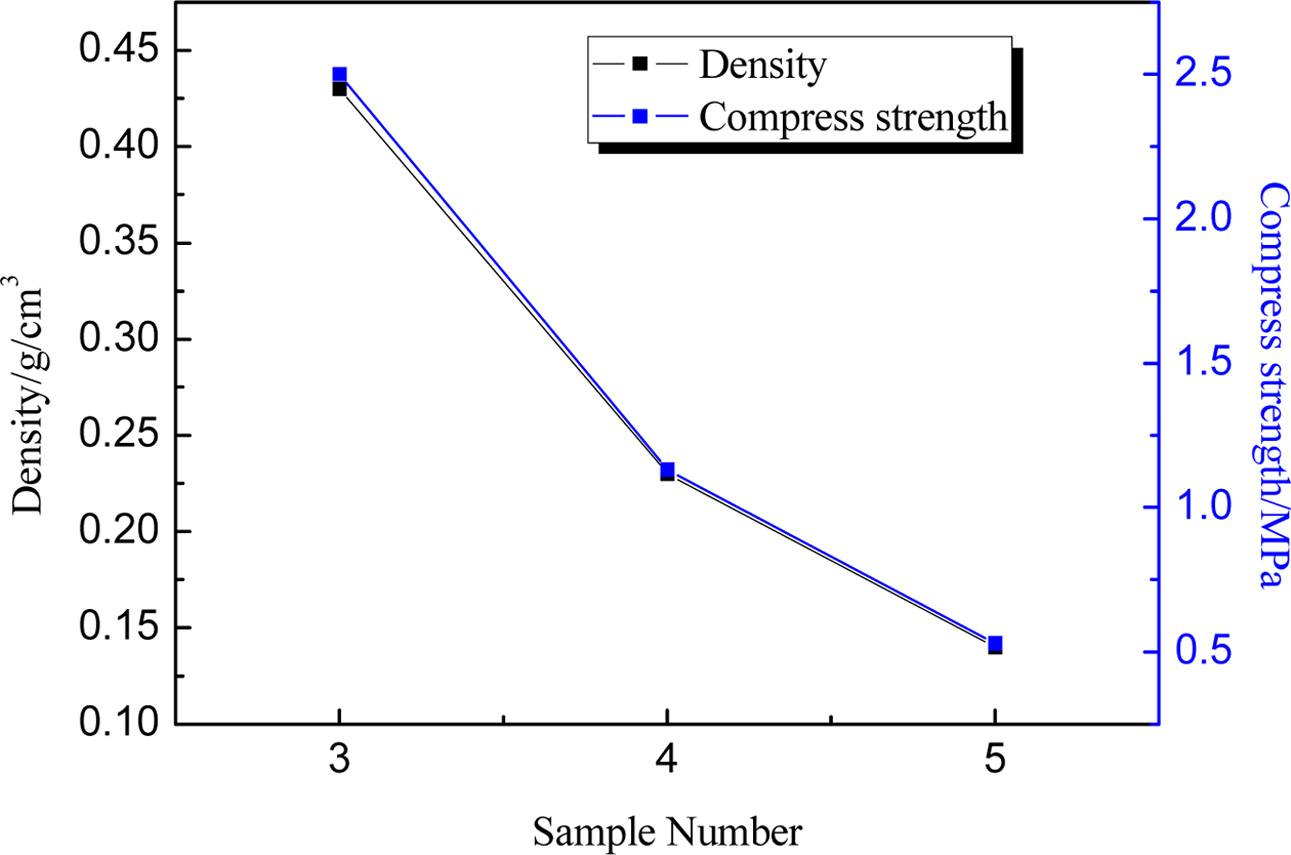
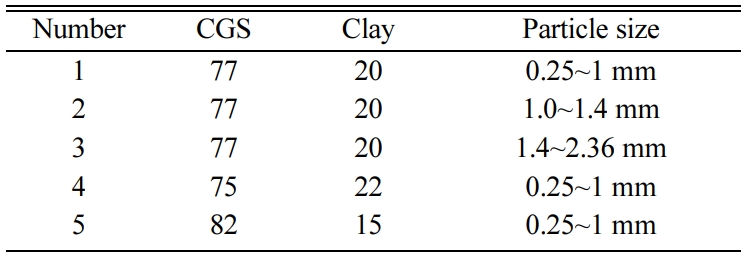
The effect of ratio of CGS to
clay
Based on the above research, the sample prepared with the
particle grain size being in the range from 0.25 to 1mm at 1,160 ºC was
relatively good. Furthermore, in order to determine the utilization of CGS, the
effect of the content of the CGS was investigated as well. For sample 3 to 5,
with different content of CGS sintered at 1,160 ºC for 2 h, the volume density
and compressive strength were detected. As displayed in Fig. 5, the value of
density and compressive strength decreased with the ratio of CGS to clay
raised. For CGS, there were amount of glass phase, and they could transform into
liquid phase at a higher temperature, which promoted oxidation of
SiC and resulted in generating more pore in samples; Meanwhile, as shown in
Table 1, the clay contained more content of SiO2 that can increase
the viscosity and associativity of the reaction system. Therefore, when the
ratio of CGS to clay increased, the samples become loose, and the value of
density and compressive strength decreased. However, according to the
literature [37], the foam ceramic can be used as walling material, it must be
the value of the density less than 1 g/cm3 and the compress strength
no less than 0.6 MPa. And at the same time, given the environmental benefit,
the utilization of CGS should be as high as possible. Hence, by contrast, when
the ratio of CGS to clay: 77:20 was relatively reasonable.
Discuss
on the optimal solution
In order to verify the feasibility of this foam ceramic as a new inorganic building
insulation material. Seen from 3.1 and 3.2, the sample with ratio of CGS to
clay (77:20), the particle grain size being the range from 0.25 to 1 mm, were
relatively good, so the repeated experiment
of the sample was carried out with the various temperature
(1,140~1,160 ºC). And the performance of the products such as volume
density, compressive strength, thermal conductivity, phase composition and
microstructure were all characterized systematically.
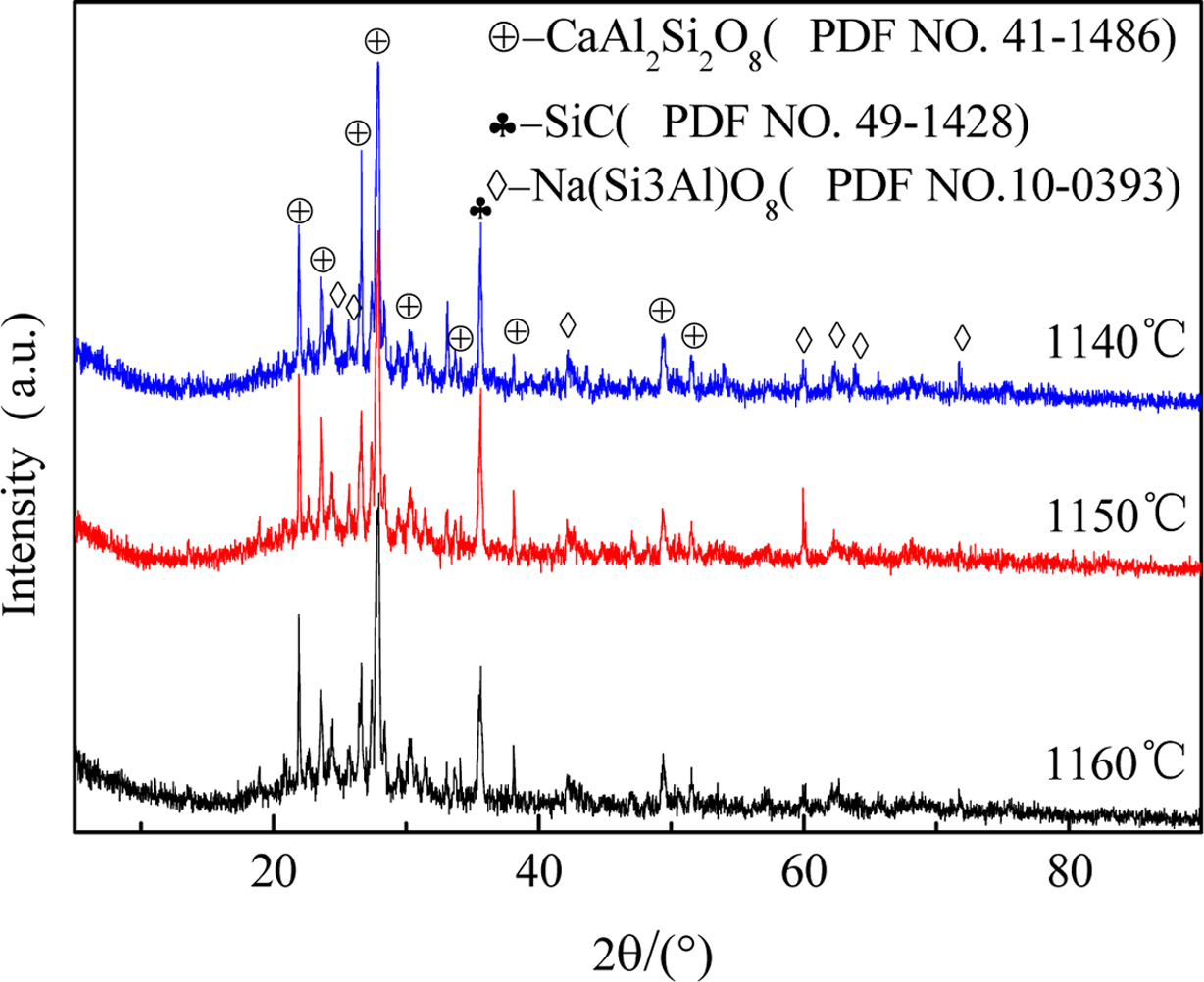
Fig. 6 depicts phase composition of the sintering products
at various temperature. It can be found that many
crystalline minerals, such as anorthite (CaAl2Si2O8),
albite (Na (Si3Al) O8), and SiC exist in all the products.
Among them, the anorthite, as a typical ceramic phase, is formed by
aluminosilicate at high temperature, which plays a key role in compress
strength of the products. In addition, as displayed in Fig. 6, the content of
SiC decreased with the sintering temperature increased, it
indicated that, as the temperature increased, the oxidation of
SiC in side became more and more complete. Hence, the result of XRD analysis is
consistent with the part of 3.1.
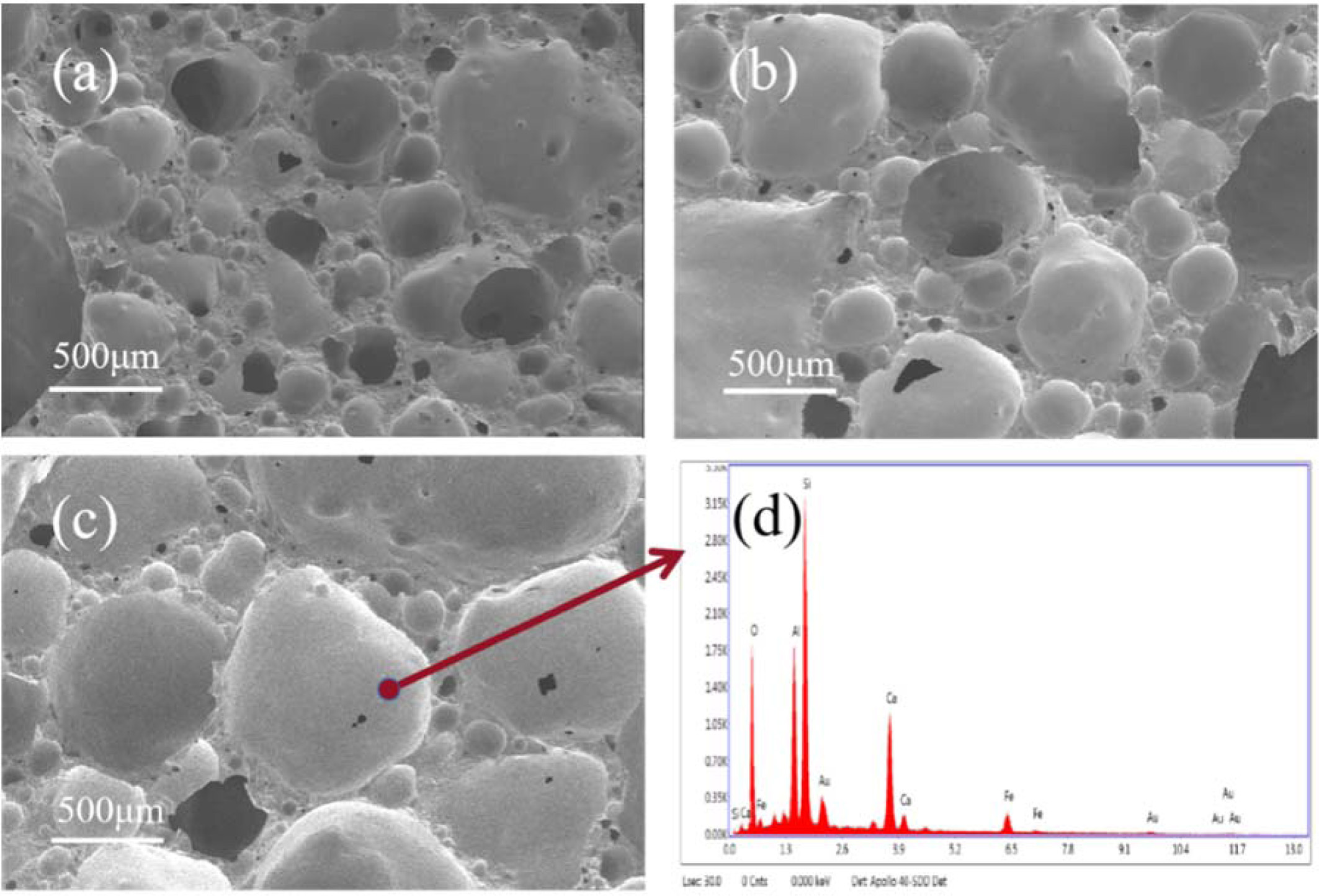
And Fig. 7 clearly revealed the evolution of micro- structure of
the products sintered at various temperatures from 1,140
to 1,160 ºC. As presented in Fig. 7, almost all the pores showed roundness and
closed in product at 1,160 ºC. And there were also relatively more
interconnected porosity in product at 1,140 ºC and 1,150 ºC. It can
be indicated that the liquid phase got more generated and viscosity of the
liquid phase decreased with sintering temperature increased [38, 39]. Thus, the
liquid phase will approach particles in ceramic body owing to surface energy.
Hence, the open porosity reduces [40-42]. Besides, the pore became significantly
larger with the temperature increased. It demonstrated that the surface tension
of the gas which generated from the oxidation of SiC caused the pores
coalescing. By contrast, the content and the size of the closed pores were the
most suitable for applying to thermal insulation material.
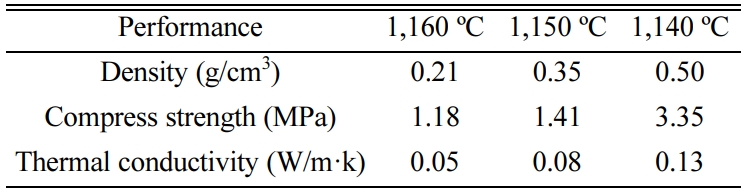
As indicated in Table 4, the density,
compress strength and thermal conductivity of the products with 77% CGS sintered at various temperatures were investigated. Table 4 illustrated that the density,
compress strength and thermal conductivity of
the products decreased with the temperature
decreased. Especially, When the product fabricated with 77% CGS sintered at
1,160 ºC, the thermal conductivity was up to
0.05 W/m·k. According to the literature [43,44], compared with organic thermal
insulation material, inorganic thermal insulation material has the greatest
advantage of being non-flammable burning, however, because of the disadvantage of the inorganic thermal insulation
materials, such as high water absorption, high cost, heat preservation performance slightly poor, etc, the inorganic thermal
insulation materials is difficult to be generalized in a large scope. As we
know, for high efficiency thermal insulation material, thermal conductivity is
less than 0.05 W/m·k, and for light insulation
material, its thermal conductivity is less than 0.23 W/m·k [45]. Thus, the sample of this paper
was fabricated by solid waste, which would not only
overcome the feature of high cost for inorganic thermal insulation materials, but
also process the virtue of lightweight and low thermal conductivity
that close to that of high-efficiency insulation material.
Hence, the samples can be expected to apply to light insulation partition panel.
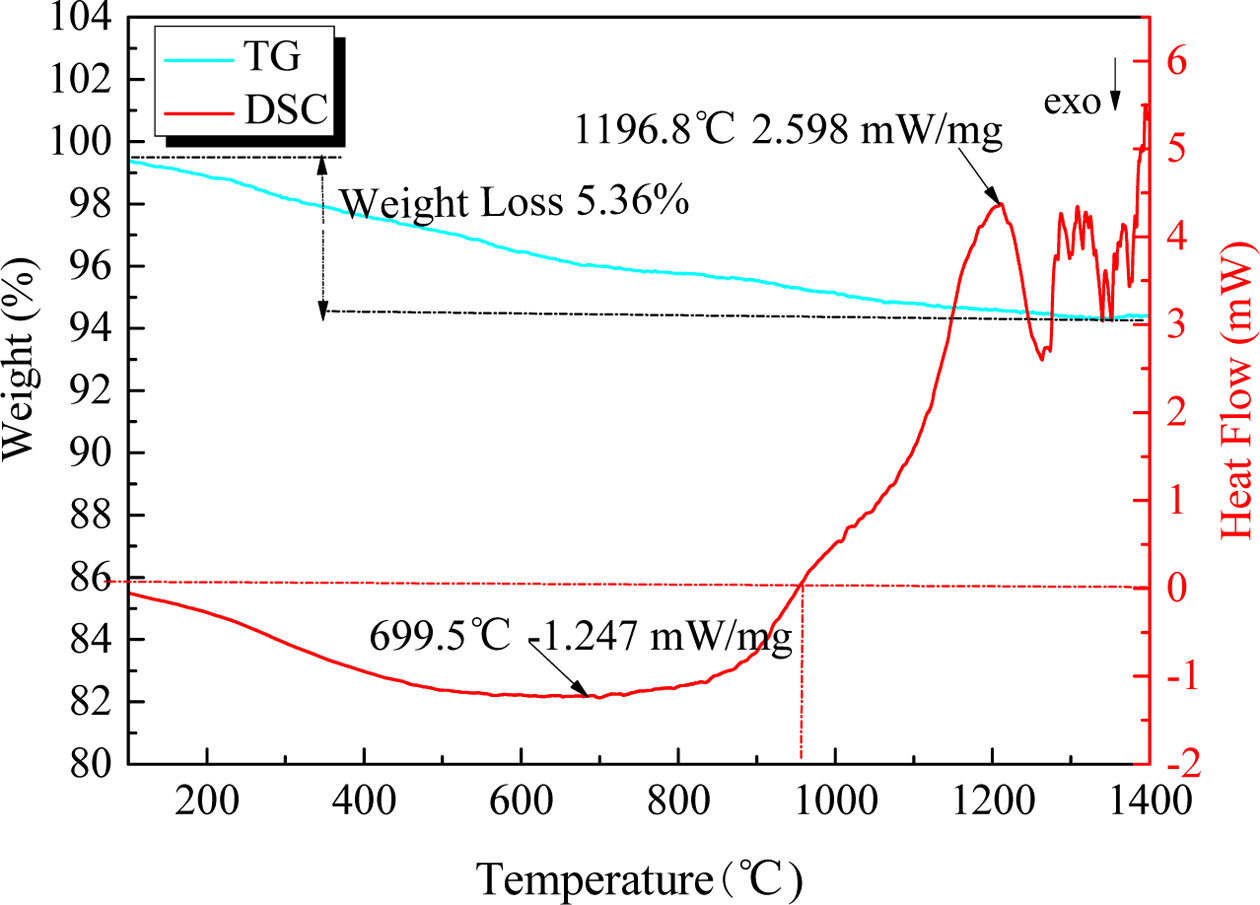
|
Fig. 2 The TG-DSC result of the mixed raw materials. |
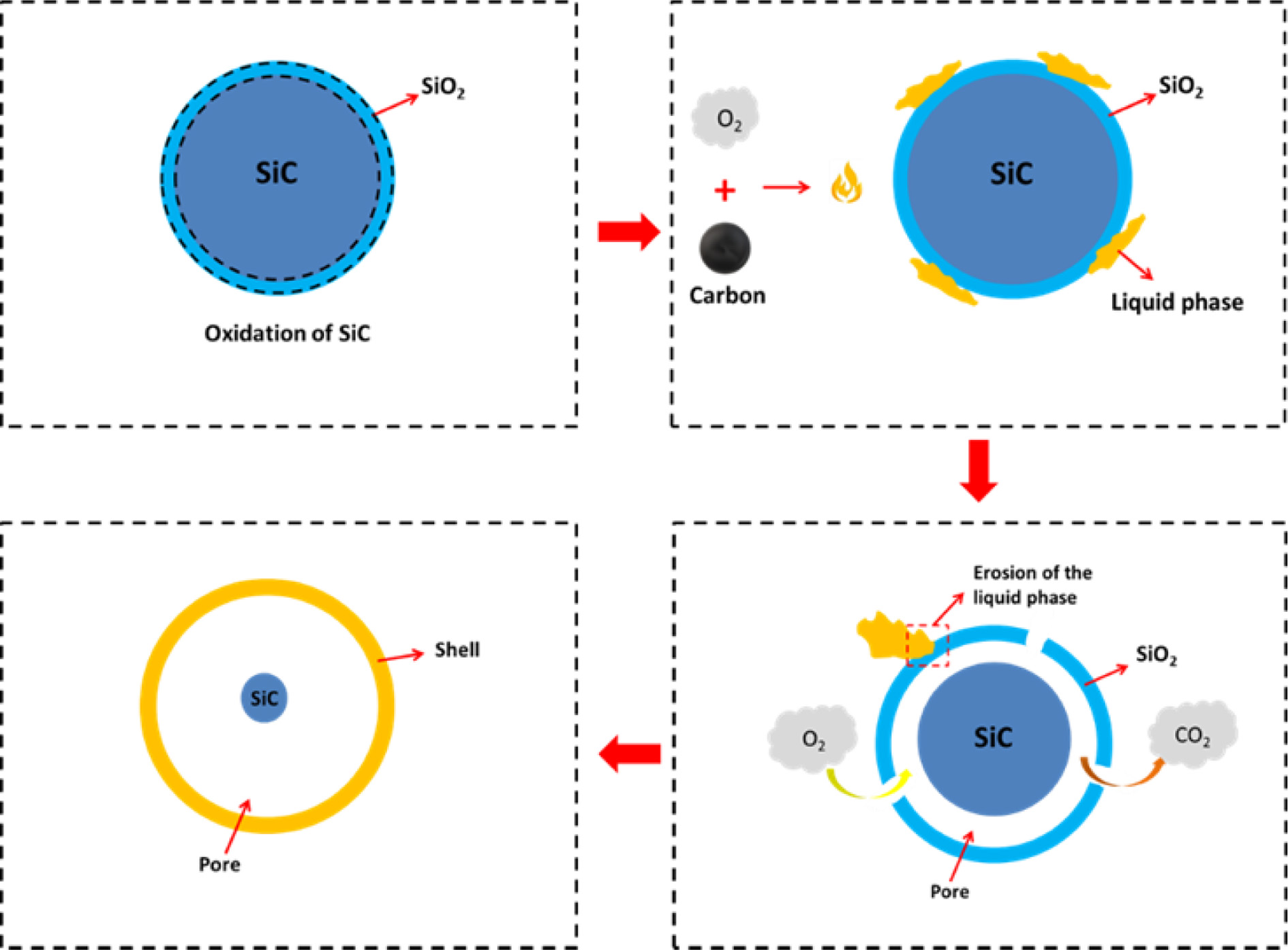
|
Fig. 3 Foaming mechanism of the SiC based on CGS. |
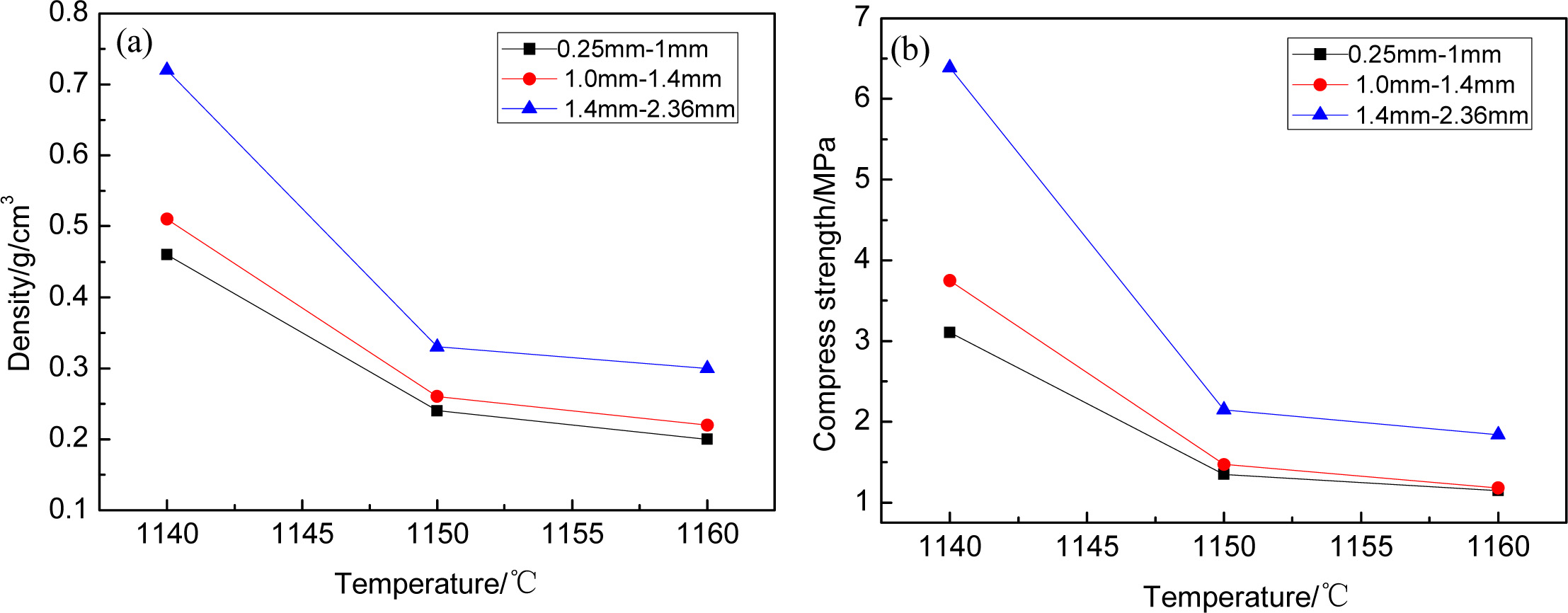
|
Fig. 4 The density and compress strength of samples with the varied grain size and temperature. |
|
Fig. 5 The density and compress strength of samples with ratio of
CGS to clay 3(72:25), 4(77:20), 5(82:15) |
|
Fig. 6 X-ray diffraction of sintering products. |
|
Fig. 7 SEM and EDX (d) image of samples with different sintering temperatures: (a) 1,140 ºC; (b) 1,150 ºC; (c) 1,160 ºC. |
|
Table 4 The performance of the products sintering various
temperature (1,140~1,160 ºC). |
In conclusion, this research emphasized the viability of
recycling CGS from chemical plant to produce foam ceramic new wall material
using SiC as foaming agent. It can be indicated that: the optimum particle size
rang was 0.25~1 mm, at the sintering temperature was 1,160 ºC, the foam ceramic
new wall material with 77% CGS presented relatively low volume density of 0.21
g/cm3, low heat conductivity coefficient of 0.05 W/(m·k), and the
corresponding compress strength of 1.18 MPa. Moreover, the total amount of CGS
is up to 77%, which greatly reduces the cost of raw materials and has played
a very beneficial role in environmental protection. Finally, it
will have great potential in the application in the field of new building materials.
This work was financially supported by the Joint Funds of
the National Natural Science Foundation of China (No. U1261118)
- 1. T.E. Peng, H.C. Li, Y.L. Liu, Y.L.Wang, and L. Liu, Ceramics 12 (2019) 00-00.
- 2. X.J. Mao, S.W. Wang, and J.Z. Daojing, in Proceedings of the 2006 Beijing International Materials Week, June 2006, edited by S. Long, X. Zhang, Y. Han, C. Peng, Y. Lu (Trans Tech Publications Limited, 2007) p.00.
- 3. L. Chen, Y. Li, and W. Liu, J. Ceram. 4 (2011) 116-120.
- 4. X. Liu, H. Li, X. Gao, X. Li, L. Wang, H. Duan, and X.G. Li, Chemical Industry Progress 11 (2012) 167-172.
- 5. Y.F Dong, X.Y. Wang, Z.H. Li, S. Liu, and C.C. Liu, Ceramics 7 (2007) 54-56.
- 6. F.F Jiao and G.Y. Zhu. Ceramics 8 (2007) 9-11.
- 7. A. Pokhrel, J.G. Park, S.M. Park, and I.J. Kim, J. Ceram. Process. Res. 14[4] (2013) 502-507.
- 8. J.G. Park, A. Pokhrel, S.D. Nam, and W. Zhao, J. Ceram. Process. Res. 14[4] (2013) 508-512.
- 9. L. Li, in “Preparation and properties of CWS/silicate foaming materials” (Qingdao University Press, 2017) p.00.
- 10. A.M. Bernardin, M.J.D. Silva, and H.G. Riella, J. Mater. Sci. Eng. A 437[2] (2006) 222-225.
-

- 11. H.R. Femandes, D.U. Tulyaganov, and J.M.F. Ferreira, Ceram. Int. 35[1] (2009) 229-235.
-

- 12. J. Luyten, S. Mullens, J. Cooymans, A.M. De Wilde, I. Thijs, and R. Kemps, J. Eur. Ceram. Soc. 29[5] (2009) 829-832.
-

- 13. Z.N. Ye, Y.W. Wang, H. Jiang, N. Li, and S.Q. Liu, Key Eng. Mater. 575-576 (2013) 461-464.
-

- 14. A. Acosta, M. Aineto, I. Iglesias, M. Romero, and J.M. Rincón, Mater. Lett. 50 (2001) 246-250.
-

- 15. V. Choudhry, S. Kwan, and S.R. Hadley, in “Utilization of lightweight materials made from coal gasification slags; Technical Report” (Praxis engineers, 2001) p.00.
-

- 16. A. Acosta, I. Iglesias, M Aineto, M. Romero, and J.M. Rincón, J. Therm. Anal. Calorim. 67 (2002) 249-255.
-

- 17. M. Aineto, A. Acosta, J.M. Rincón, and M. Romero, Fuel 85 (2006) 2352-2358.
-

- 18. N.J. Wagner, R.H. Matjie, J.H. Slaghuis, and J.H.P. van Heerden, Fuel 87 (2008) 683-691.
-

- 19. T. Wu, M. Gong, E. Lester, F.C. Wang, Z.J. Zhou, and Z.H. Yu, Fuel 86 (2007) 972-982.
-

- 20. S.Q. Xu, Z.J. Zhou, X.X. Gao, G.S. Yu, and X. Gong, Fuel Process. Technol. 90 (2009) 1062-1070.
-

- 21. W.J. Song, L.H. Tang, X.D. Zhu, Y.Q. Wu, Z.B. Zhu, and S. Koyama, Fuel 89 (2010) 1709-1715.
-

- 22. W.J. Song, L.H. Tang, X.D. Zhu, Y. Wu, Y. Rong, Z. Zhu, andS. Koyama. Fuel 88 (2009) 297-304.
-

- 23. G.S. Yu, Q.R. Zhu, G.Z. Chi, Q.H. Guo, and Z.J. Zhou, Fuel Process. Technol. 104 (2012) 136-143.
-

- 24. L.X. Kong, J. Bai, Z.Q. Bai, Z. Guo, and W. Li, Fuel 108 (2013) 76-85.
-

- 25. Y. Tang, H.F. Yin, H.D. Yuan, H. Shuai, and Y.L. Xin, Adv. Powder Technol. 27 (2016) 2232-2237.
-

- 26. M.J. Du, J.J. Huang, Z.Y. Liu, X. Zhou, S. Guo, Z.Q. Wang, and Y.T. Fang, Fuel 224 (2018) 178-185.
-

- 27. H.F. Yin, Y. Tang, J.Z. Zhang, and J. Chin, Ceram. Soc. 39 (2011) 233-238.
- 28. L. Ding, W. Ning, Q.W. Wang, D.N. Shi, and L.D. Luo, Mater. Lett. 141[15] (2015) 327-329.
- 29. X. Chen, A. Lu, and G. Qu, Ceramics International 39[2] 92013) 1923-1929.
-

- 30. H. Wang, Z. Chen, L.L. Liu, R. Ji, and X.D. Wang, Ceram. Int. 44 (2018) 10078-10086.
-

- 31. Chinese Standard, No. GB/T5486-2008 (2008) p.00.
- 32. X. Xi, L. Xu, A.Z. Shui, Y.M. Wang, and M. Naito, Ceram. Int. 40 (2014) 12931-12938.
-

- 33. X. Xi, A. Shui, Y.F. Li, Y.M. Wang, H. Abe, and M. Naito, J. Eur. Ceram. Soc. 32 (2012) 3035-3041.
-

- 34. T.W. Cheng and Y.S. Chen, Ceram. Int. 30 (2004) 343-349.
-

- 35. B. Chen, K. Wang, X.J. Chen, and A.X. Lu, Mater. Lett. 79 (2012) 263-265.
-

- 36. H. Wang, M. Zhu, Y.Q. Sun, R. Ji, L.L. Liu, and X.D. Wang, Constr. Build. Mater. 155 (2017) 930-938.
-

- 37. Y.P. Feng, H.F. Yin, H.D. Yuan, L. Zhang, and H. Cui, Silicate Bulletin 3 (2014) 55-59.
- 38. H. Shuai, H.F. Yin, and H.D. Yuan, Coal Convers. 38 (2015) 44-48.
- 39. H.D. Yuan, H.F. Yin, Y. Tang, H. Shuai, and Y.L. Xin, Materials 13[6] (2020) 1346.
-

- 40. S. Kumar, K.K. Singh, and P. Ramachandrarao, J. Mater. Sci. 36[24] (2001) 5917-5922.
-

- 41. F.J. Torres, E.R.D. Sola, and J. Alarcón, J. Eur. Ceram. Soc. 26[12] (2006) 2285-2292.
-

- 42. T.K. Mukhopadhyay, S. Ghosh, J. Ghosh, S. Ghatak, and H.S. Maiti, Ceram. Int. 36[3] (2010) 1055-1062.
-

- 43. Q.W. Zhu, F.D. Wu, and J.P. Zhao, New Build. Mater. 6 (2012) 12-16.
- 44. M.S. Al-Homoud, Build. Environ. 40[3] (2005) 353-366.
-

- 45. B.P. Jelle, Energy Build. 43[10] (2011) 2549-2563.
-

 This Article
This Article
-
2020; 21(6): 640-646
Published on Dec 31, 2020
- 10.36410/jcpr.2020.21.6.640
- Received on Apr 3, 2020
- Revised on Jun 19, 2020
- Accepted on Jul 16, 2020
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Funding
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hongfeng Yin
-
Provincial Experimental Teaching Demonstration Center for Functional Materials, College of Materials Science & Engineering, Xi’an University of Architecture & Technology, 710055 Xi’an, China
Tel : +86 29 82205245 Fax: +86 29 82205245 - E-mail: yinhongfeng@xauat.edu.cn







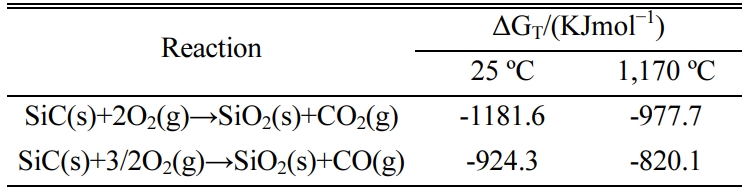
 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.