- Effect of (MgO/CaO) molar ratio on glassy phase viscosity and pyroplastic deformation in floor tiles
Neslihan Tamsu Selli*
Department of Materials Science and Engineering, Gebze Technical University, 41400 Gebze, Kocaeli, Turkey
In this study, effect of MgO/CaO
molar ratio on the floor tiles properties was investigated. The viscosity of
the bodies was successfully calculated by fleximeter analyses data. High
MgO/CaO ratio causes enhancement of the densification temperatures and
dissolution of the crystals in the floor tile body. The results showed a,
during firing, high MgO/CaO ratio up to 6,505 value, formed, low viscosity
liquid phase and allowed low sintering temperature approximately 20 oC
when compared to the standard floor tile body. For YK_3 composition will be
evaluated due to has allowable deformation amount for the production. This
floor tile composition will be successfully produced in production line.
Keywords: Floor tile, Deformation, Viscosity, Sintering
Ceramic floor tiles that are fired at high temperatures
such as 1,150-1,180 oC for 30-45 min and have water
absorption below 3%. Floor tiles can be produced either glazed
or unglazed, or they can be produced as coloured body
by adding color pigments to their initial compositions. Ceramic
floor tiles are partially the vitrified product of mixtures of clay, quartz
sand, and feldspar, after heat treatment at temperatures in range
1,150-1,180 oC. The primary purpose of these three components
may be described as follows [1-3]: Clays provide plasticity to the body and
provide dry strength. They gives the colour depends on their own color and
impurities. After clays, feldspars have also critical role in the ceramic tile
production sector. Feldspars are used as melting agents in the recipes. They
are reduce the sintering temperature by forming liquid phase when the ceramic
body is firing. In addition to the these raw materials which have plastic and
melting properties in ceramic structures, there is a need for a non-plastic
filler which will keep the body integrity in high temperature ranges. Quartz
acts as a skeleton in the body. It is the main filler which is incorporated
into the ceramic body as a real component or as a component from clay and
feldspar. It constitutes the roughest particle size part of the structure. The
large grain size interval provides resistance to cracks during drying and
prevents deformation by forming a skeleton during firing [4, 5]. Maiti et al.
[6], reported that during firing, tile body assists
dissolution of quartz, recrystallization of secondary
mullite and reduction of closed pores in the tile body. It has been also
reported by Bull [7], alkaline earth materials in the tile composition resulted
in the speedy glassy phase formation during fast firing cycle. During fast
firing cycle, for some body compositions, glassy phase viscosity decreases
suddenly and it could create some distortions and deformations on the sample
during firing. Pyroplastic deformation is defined as deformation which occurs
the effect of product weight during high temperatures [8]. By understanding the
compositon effect on melting behaviour of the ceramic body, it can
be understood that pyroplastic deformation, reactions
and mechanical properties during fast firing [5]. Öztürk et al. [9]
investigated the effect of alkaline oxides on porcelain tiles using factorial
design method. MgO, CaO, Na2O and K2O were used in the new compositions. It was
found that the strength increased, and water absorption decreased by means of
alkaline oxide variation in the porcelain tile body. Rastelli et al. [10],
studied effects of spodumene and zirconium based materials on the reology of
the porcelain tile slurry. Nevertheless, in this study effect of these raw
materials on the sintering behaviour body did not mentioned. n
this study, the effect of these compositions on glassy
phase viscosity on wall tile was not investi- gated and only wall tile was taken into
consideration. Elmas et al. [11], studied about the effect of boric acid and
lithium carbonate scombination on sintering and microstructure of single-fired
wall tile bodies. They found that combination of the lithium-carbonate and
boric acid provided low sintering temperature, and high strength values for
single fired wall tiles. Sousa et. al. [12], investigated sintering behavior of
porous wall tile bodies during fast single firing process. In this study, wall
tile composition had red clay, limestone and quartz. Its technical properties
and microstructure was evaluated considering the different
sintering temperatures of the wall tile body.
Many studies about glassy
phase viscosity of ceramic bodies have been reported. Porte et al. [13],
studied about creep viscosity of vitreous china composition containing
different mullite and quartz cystals. They used four point bend rig for creep
measurement. However, they did not report any information about pyroplastic
deformation relation with glassy phase viscosity. Tuna et al. [14], studied influence of
porcelain tile starting composition
containing spodumene on the pyroplastic deformation evolution during firing.
They found that pyroplastic deformation was decreased with increasing Li2O
addition in porcelain stoneware tiles. Tuncel et al. [8], investigated effect
of different Na2O/K2O and SiO2/Al2O3
ratio affects the viscosity of the system as the amount of mullite increases.
Other oxides that affect the vitreous phase viscosity in the ceramic bodies are
magnesium oxide and calcium oxide. These oxides are generally used for allow
the tiles firing at short firing cycle (24-30 min at 1,150-1,180 oC)
and increasing sintering rate in the ceramic bodies. Dondi et al. [15],
reported that magnesium accelerate the sintering
and decrease the ripenning temperature of the body and with this way narrowed
the firing interval of the body. On the
other hand, magnesium containing porcelain stoneware tile compositions shows
lower mechanical strength due to increasing porosity. Mukhopadhyay et al. [16],
investigated the effect of talc component in illitic clay composition on
thermal, mechanical properties and microstructure of the bodies. Talc is shown
as 3MgO.4SiO2. H2O formula and it is magnesium silicate
structure. It was observed that talc/feldspar
composition reduced the vitrification temperature. MgO, which is incorporated
by addition of talc provides more
glassy phase formation with low glassy phase viscosity. Biasini et al. [17],
designed porcelain tile composition containing different talc and chlorite
components. The occurance of
magnesium silicates does not affect the technological properties of
semi-finished product, but it
influences remarkably the firing behaviour. However, in these studies, the effect of glassy phase viscosity on
pyroplastic deformation was not specified for floor tiles. Consequently, the
research objectives of this study were the first to develop and understand
about how viscosity of ceramic floor tile body changes as a function of
alkaline earth oxides molar ratio, (MgO/CaO), the second objective was to
investigate effect of viscosity on
pyroplastic deformation of ceramic floor
tiles an also understanding this compositional change on microstructure of the
tile bodies.
Starting from the standard ceramic floor tile body mix,
different amounts of talc and calcite were added into the system. New
compositions were denoted as YK_1, YK_2, YK_3 and YK_4. Chemical
characterization was carried out by means of wavelength dispersive
X-ray fluorescence spectrometry (XRF), using a Philips model PW 2400 XRF
instrument fitted with an Rh white fluorescent tube. The samples were prepared
as fused beads using a Philips PERL’X3 instrument. Chemical analyses of the
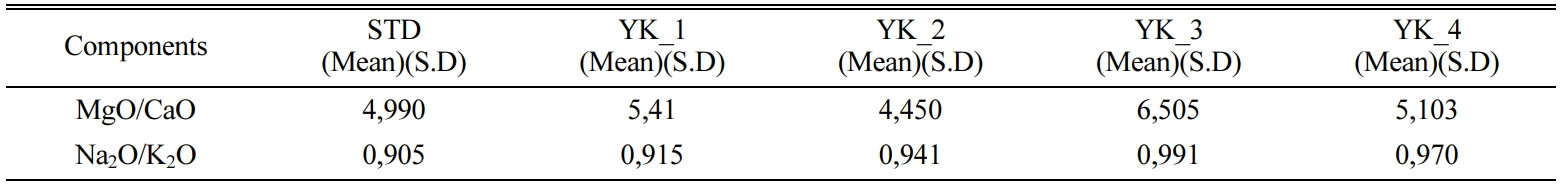
compositions are shown in Table 1. Seger formulation was applied to prepare new
compositions and MgO/CaO molar ratio was chosen as the main
variable parameter to prepare the compositions and amount of
total molar alkaline oxides (Na2O and K2O) was kept approximately constant in
the com- positions. Seger
formulation details of the compositions are given Table 2. New compositions
were wet milled by a laboratory ceramic jar mill containing 70 wt.% solid and
1.0 wt.% deflocculant, for 20 min. Slips were dried at 110 oC.
Samples (50 ´ 50 ´ 6 mm3) and (60 ´ 6 ´ 6 mm3) were hydraulically
compacted using uniaxial pressing at 300-350 kg/cm2.
The shaped samples were dried at 110-120 oC for 24 h till the
moisture content less than 0.5%.
Sintering temperatures of the compositions were determined
by flex point (i.e., temperature at which densification rate is maximum) using
the optical dilatometer (Misura 3.32, ODHT-HSM, Expert System Solutions,
Italy). Samples were heated in an optical dilatometer at a rate of 50 oC/min
up to 1,250 oC without soaking at peak temperature in air
atmosphere condition to determine flex points as stated by Paganelli
[18]. Total heat treatment was approximately 30 min and the peak temperature
was 1,160 oC in roller kiln. The
firing shrinkages were determined by measuring the diameter
of the discs before and after sintering. The water
absorption of the sintered disc was measured by a water
displacement method according to ISO 10545-3.
The colour values of the new compositions and standard
composition were measured by a spectrometer (Minolta CR,
300 Colormeter). The colorimeter operates on the
CIELab method, which is utilized technique in the ceramic production to
determine the whiteness and colour of the tiles by measuring three main
parameters (Hunter parameters) L* (brightness) from absolute white L=100 to
absolute black L=0, an (red–green), bn (yellow–blue) elaborated from the
visible spectra. The bending strength of sintered samples was measured with an
electronic universal tester (Model 5569, Instron Ltd.) by a three-point bending
test with a lower span of 50 mm and crosshead speed of 1 mm/min,
based on ASTM standard C1161-90. The crystalline phases in the fired samples
were determined by XRD analyses. For XRD analyses, sintered samples were
scanned from 2q=5 to 70o,
at a scanning speed of 2o/min, using a Rigaku Rint 2000 Series
diffractometer with Cu Ka
radiation at 40 kV and 30 mA. The crystalline phase composition
was quantatively analysed with the software Material
Analysis Using Diffraction (MAUD) based on the Rietveld method. Microstructural
observations were performed on selected fired samples using a scanning electron
microscope (EVO-50, Carl-Zeiss, Germany). Chemical etching was employed to
reveal the presence of certain crystalline phases by immersing the relevant
samples in 10% hydrofluoric acid (HF) solution at room temperature for 20 s.
Qualitative EDX (Oxford Inst. 5108 Link) analyses were
performed simultaneously with
microstructural observations in order to distinguish the various
phases. To determine the pyroplastic deformation behavior of the compositions,
rod shapes samples having (80 mm ´
7 mm ´ 7 mm)
size were produced and fired using an optical fleximeter (Misura, ODLT Flex
1400-30) with a firing regime of 25 oC/min to 1,160 oC
and waiting 5 min at the peak temperature. After fleximeter analysis, the
pyroplastic index (PI) values of samples calculated according to Eq. (1) [8].
The viscosity of the ceramic floor tile bodies (ɳ) was calculated using the
results of fleximeter analysis according to Eq. (2) [8].

where s is the total
deformation, b is the sample thickness, l is the distance between
supports, ρb is the bulk density of the body, and g is the
gravitational constant
|
Table 1 Chemical composition (wt%) of ceramic floor tiles recipes. |
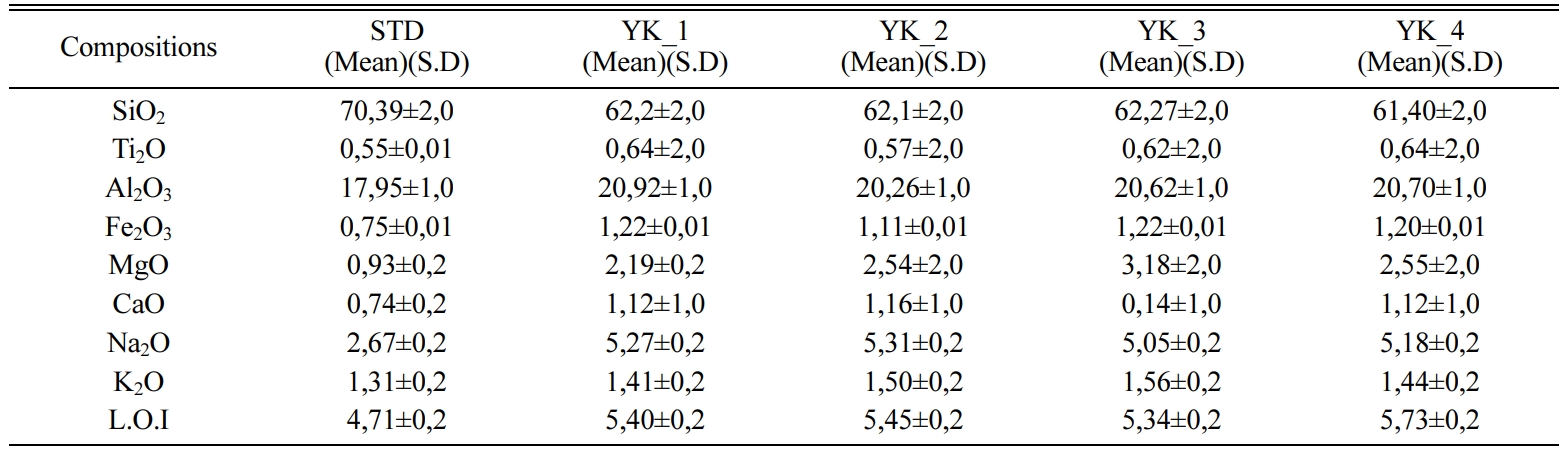
* L.O.I: Lost of ignition |
The chemical and mineralogical compositions of the tiles
affect the deformation behaviour of the tiles. For this reason, the crystalline
phase compositions of samples fired at 1,160 oC were
investigated based on XRD patterns. XRD pattern of standard composition is
given in Fig. 1. Quartz, albite and anothite were present in standard floor
tile. XRD analysis also was performed on the recipes with increasing MgO/CaO
ratio. Com- parison of the XRD
patterns of the recipes was given in Fig. 2. According to the XRD patterns, the
main crystalline phases were quartz, albite and anorthite. In order
to determine the composition effect on the crystalline
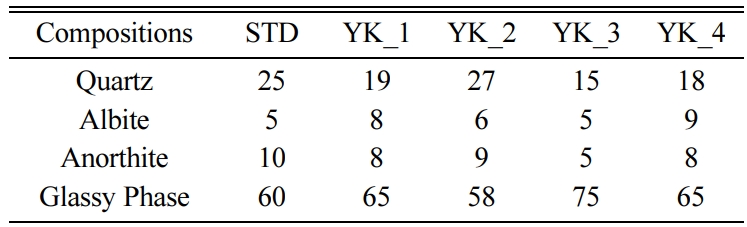
phases more clearly, amount of the crystalline phases and
glassy phase were determined. The amounts of crystalline and glassy phases
determined by the MAUD programme for all of the compositions are given in Table
3. Standard floor tile composition has 5 wt% albite, 10 wt% anorthite, 60 wt%
glassy phase. Different MgO and CaO ratio affected the phase content of the
floor tile bodies. When the MgO/CaO molar ratio reached 6,505 in YK-3, the
amount of the quartz decreased compared to the STD, and the amount of albite
remained constant and there was a decrease in anorthite crystals amount. The
increase in the MgO/CaO ratio in YK-3 composition also affected the glassy
phase viscosity. The amount of deformation during firing was determined and
viscosity values were calculated according
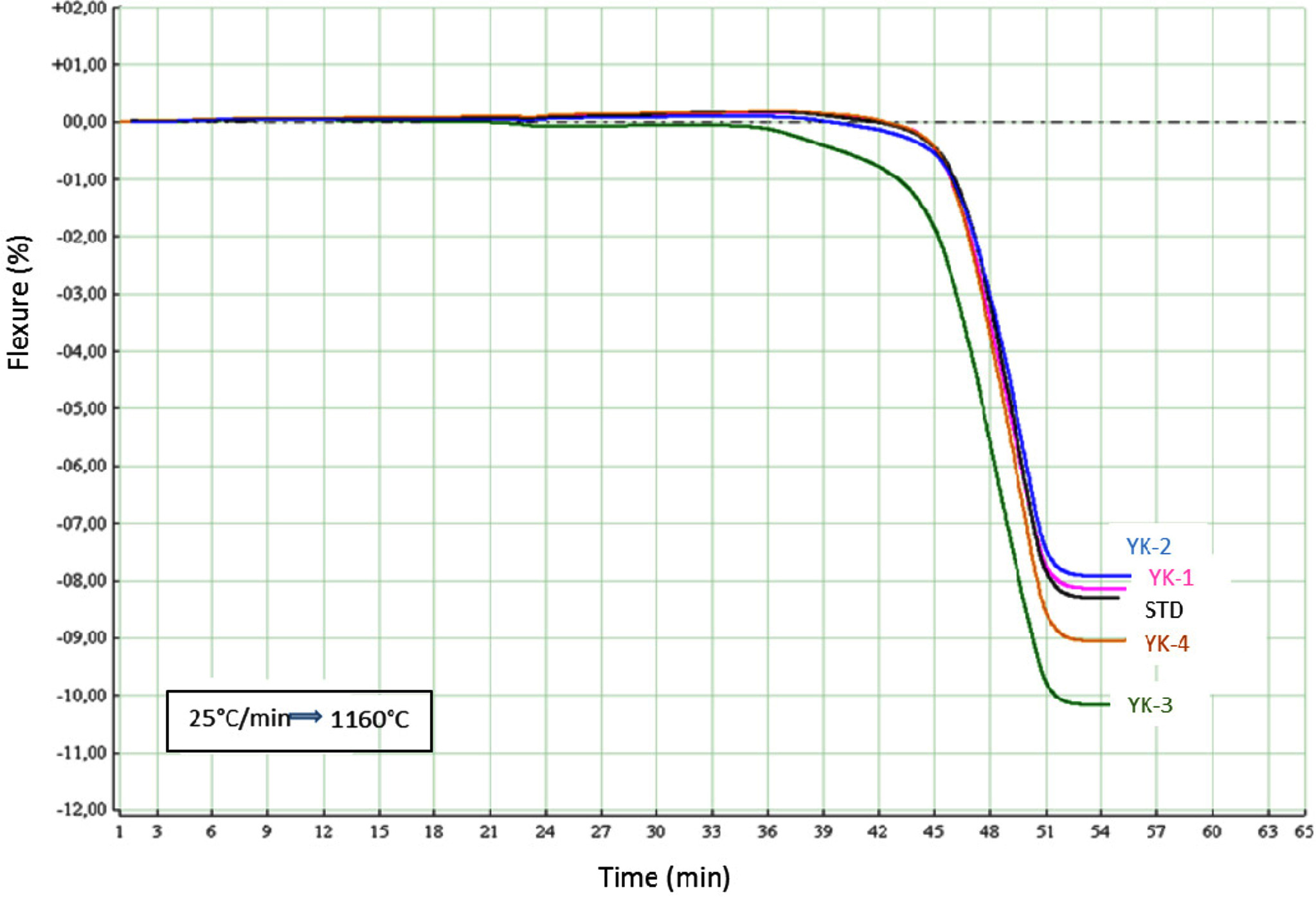
to Eq. (2) by using fleximeter. Fig. 3 shows the deformation behaviour of the
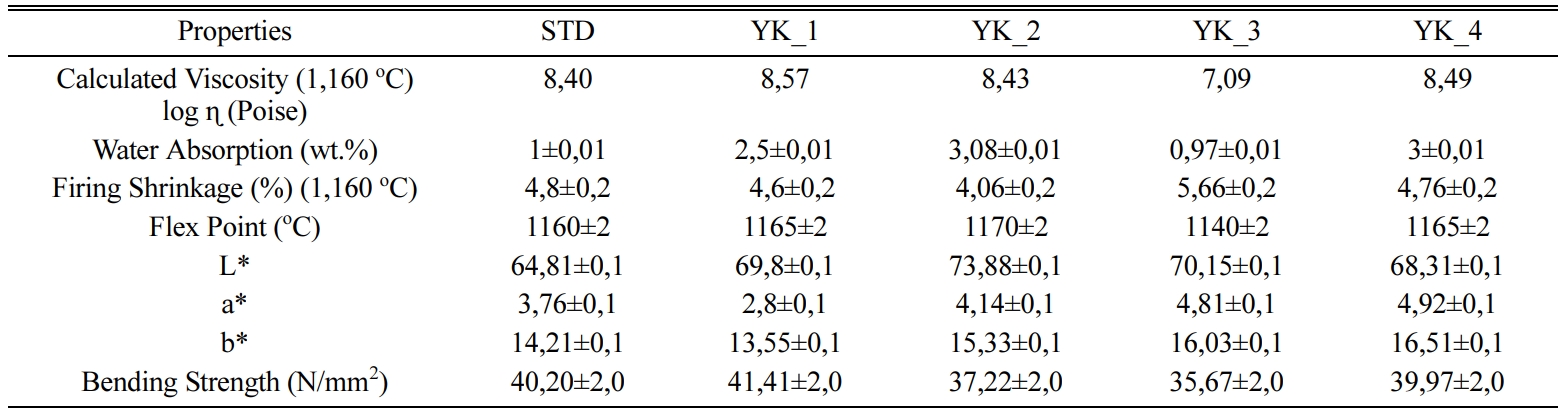
floor tiles according to firing conditions and Table 4 shows the calculated viscosity values depend on the
fleximeter analyses and technological properties of the
compositions. The viscosity value of
the YK_3 composition at 1,160 oC is 107,09 P, while the viscosity
value of the YK_2 composition at 1,160 oC is 108,43 P, it can
be showed that the crystalline phases
are more effectively dissolved. With the increase in MgO/CaO ratio in the
compositions, the increment of glassy
phase fastened dissolution of the crystalline phases when compared the
compositions have low amount of MgO/CaO ratio. When comparing deformation behaviours with fleximeter analysis (Fig. 4), especially for YK_3
composition, the sample started deformation movement after 36 min. During the
sintering period, the deformation movement of the YK_3 composition completed
movement after 51 min and total deformation amount is approximately 10,3%. For
YK_2 composition with the lowest MgO/CaO ratio, deformation movement started
after 45 min and this motion completed
after 54 min. Total deformation is
about 7.83%. YK_2 was then followed by YK_1, STD and YK_4 compositions. In
addition to this, the effect of
magnesium oxide and calcium oxide ratio on sintering was investigated by optical dilatometer analyses. In particular,
the results of the analysis of the temperature at which the samples reach their
maximum sintering point (Table 4). As MgO/CaO ratio increases, the flex point of the bodies decreases. YK_2
composition has 4,450 MgO/CaO ratio
and its flex point is approxi- mately 1,170 ± 2 oC,
while YK_3 composition has 6,550 MgO/CaO
ratio and its flex point is approximately 1,140 ± 2 oC.
This decrease in flex point is quite remarkable
in the compositions. It is evident that by increasing the MgO/CaO ratio in the
composition has a sintering accelerating effect. When investigated the
literature, an a study performed Dondi et al., about porcelain tiles, the
effect of magnesium silicates was emphasized
[15]. They stated that magnesium accelerates the sintering of porcelain tile bodies and narrows the firing range of
the body. In a study performed by Mukhopadhyay et al., the role of talc on
porcelain sintering was investigated. They observed that the talc/feldspar composition reduces the vitrification
temperature [16]. It has been
determined that magnesium oxide which is incorporated by the addition of talc,
provides more liquid formation with lower viscosity, decreases the maturation
temperature but narrows the firing range of the body. Therefore, the results of
this study are consistent with in the literature.
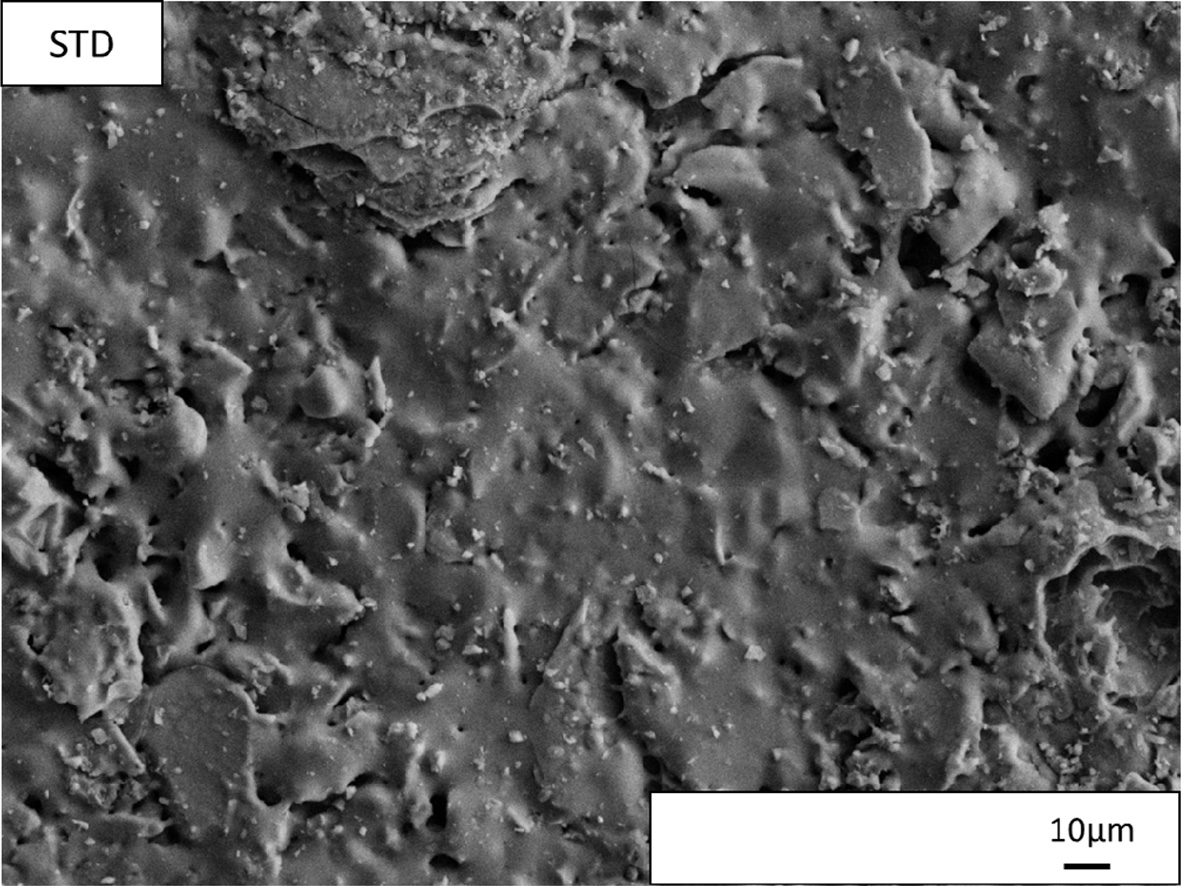
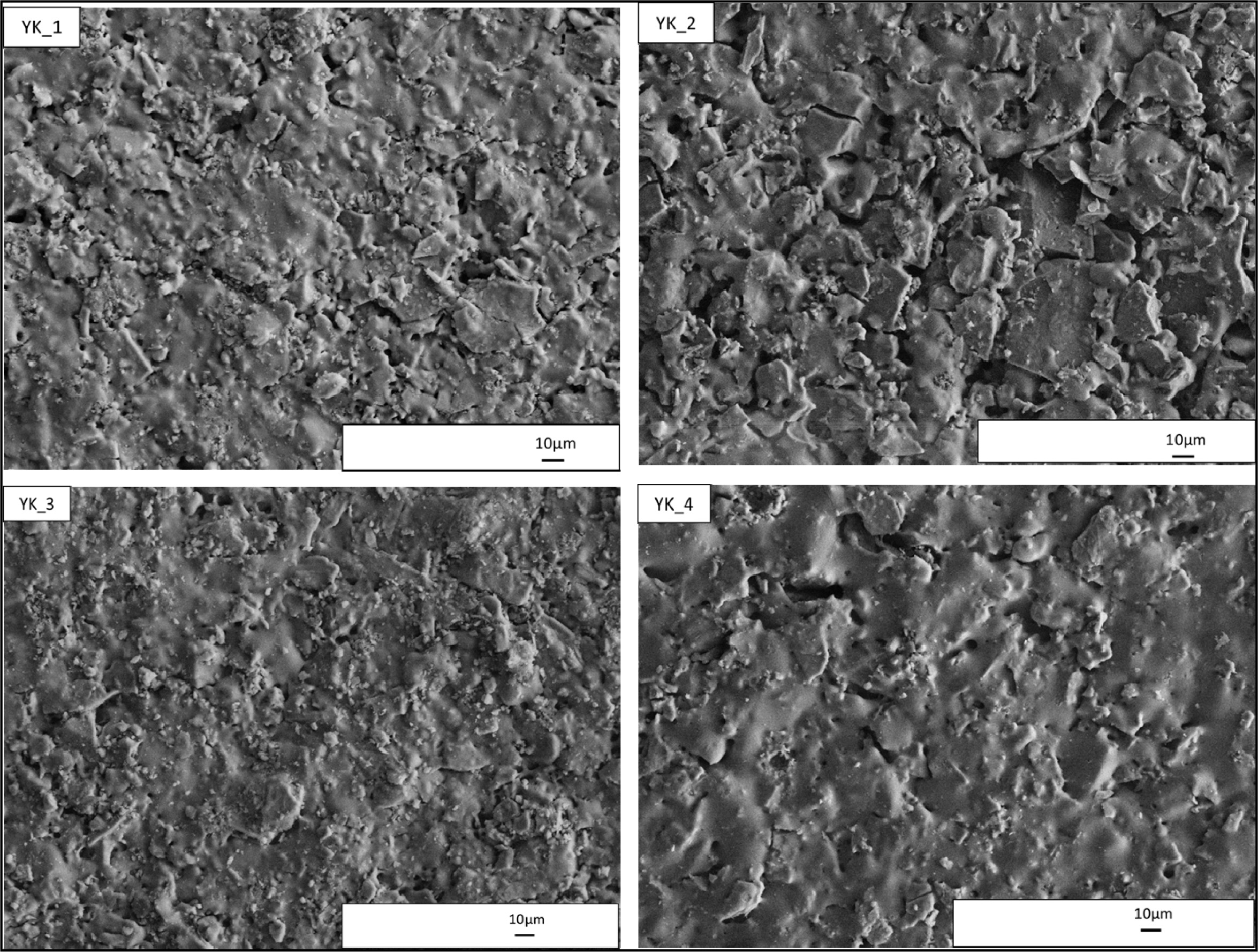
SEM images taken from the polished surfaces of the
industrially fired standard and new floor tile bodies are represented Fig. 5
and Fig. 6. When the surface micro-
structures were investigated, generally similar structures
could be seen Fig. 6. Therefore, EDX analyses of the samples were carried out
in order to see types and distributions of the crystalline
phases, pores, etc. depend on the compositions.
In addition, a typical SE (secondary electrons) image taken on the
fractured surface etched with 10%HF solution for 20 s to selectively remove the
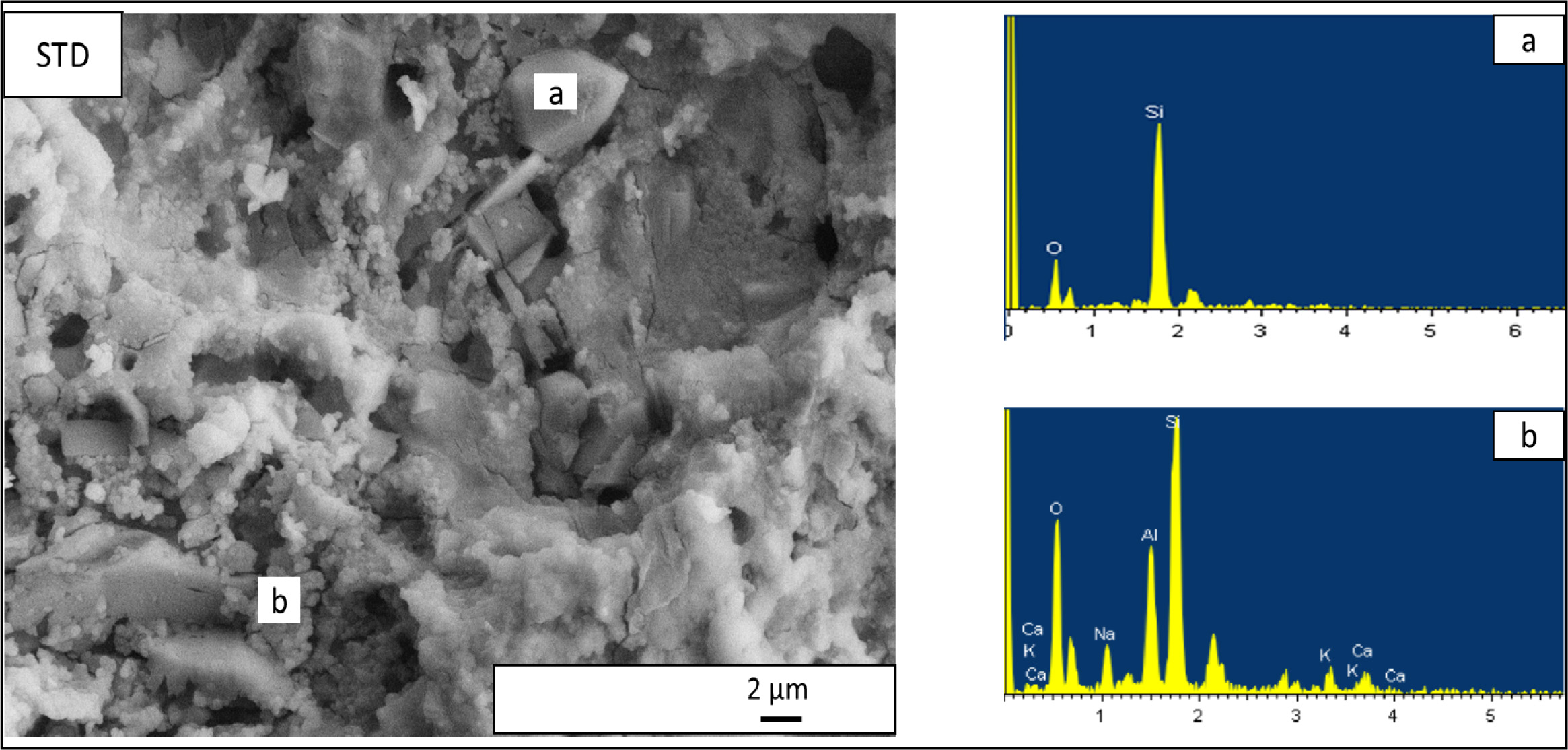
glassy matrix shows some of the constituent crystalline phases. For standard
floor tile body composition (Fig. 7), irregular shaped particles contain mainly
silicon and oxygen so being quartz crystals (Fig. 7 (a)), presence of small
spheroidal crystals forming clusters that contain mainly
calcium, sodium, aluminium, silicon and oxygen so being
attributable to anorthite and albite crystals (Fig. 7(b)). Selected
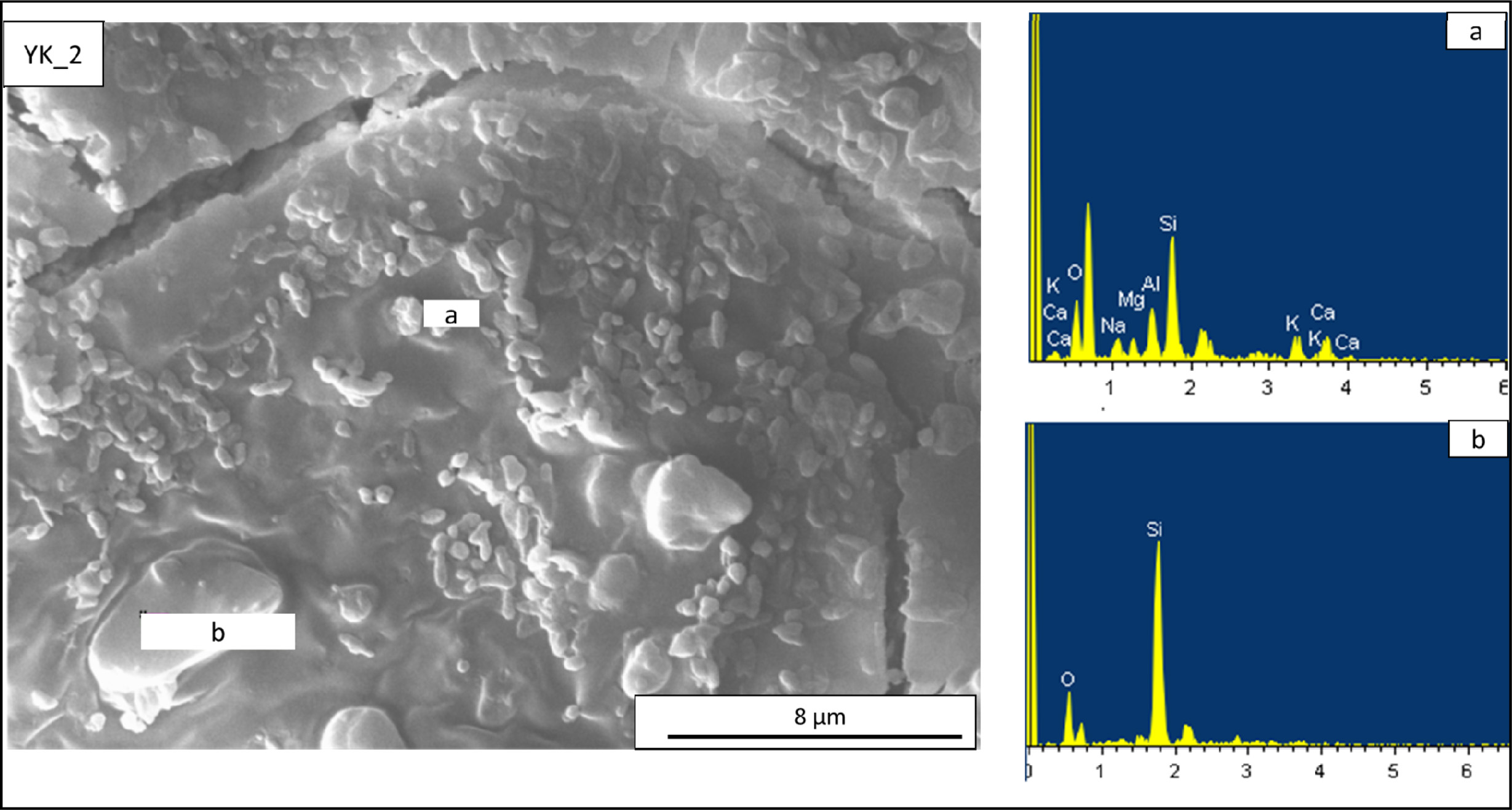
microstructures of the YK_2 and YK_3 compositions are shown in Fig. 8 and Fig.
9. The microstructure of the YK_2 composition is shown in Fig. 8. In this
composition, according to the results of (a) EDX analysis of round crystalline
clusters, it is seen that there is a region of albite and anorthite crystals
and the grain sizes are around 1-2 microns. Irregular and
large grains are also present (b), according to the EDX
analysis taken from these regions, it is seen that these grains are quartz
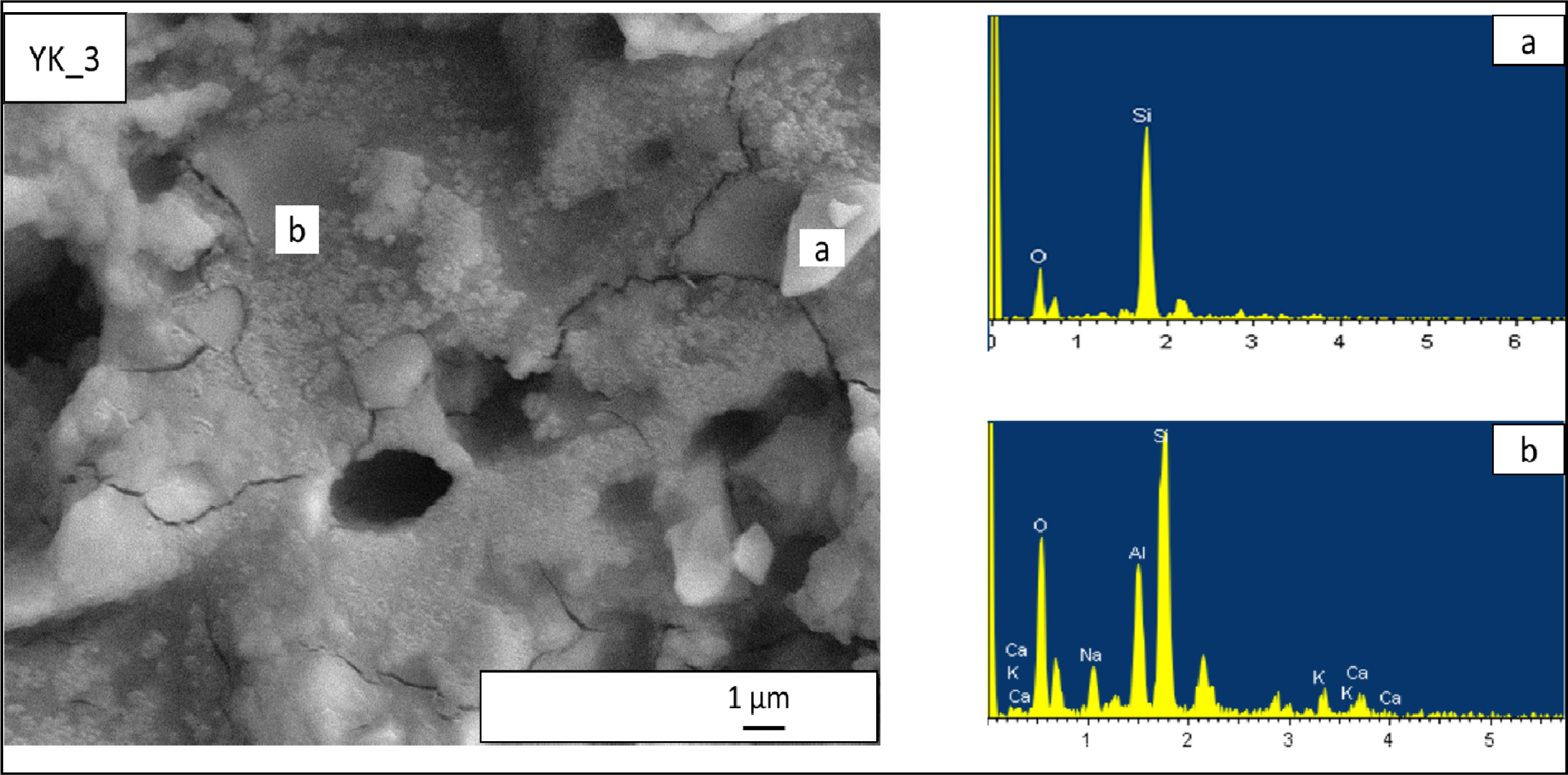
crystals. Microstructure of YK_3 composition is given in Fig. 9. This
composition also contains quartz crystals (Fig. 9(a)), albite,
anorthite (Fig. 9(b)) and but the crystalline dimensions are quite
small (400-500 nm). Less viscous liquid phase and its able to eliminate larger
pores and increase crystals dissolutions.
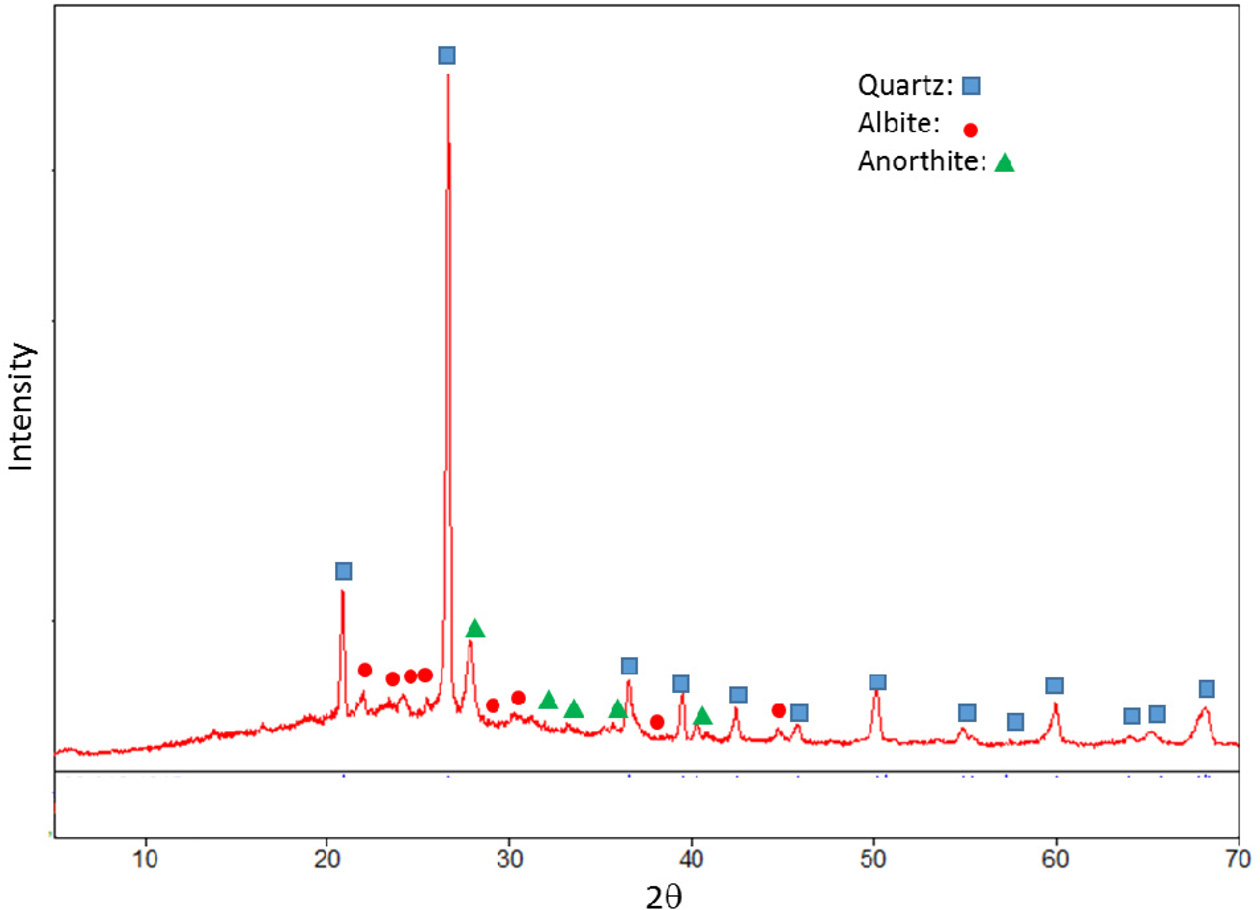
|
Fig. 1 XRD pattern of the standard (STD) composition. |
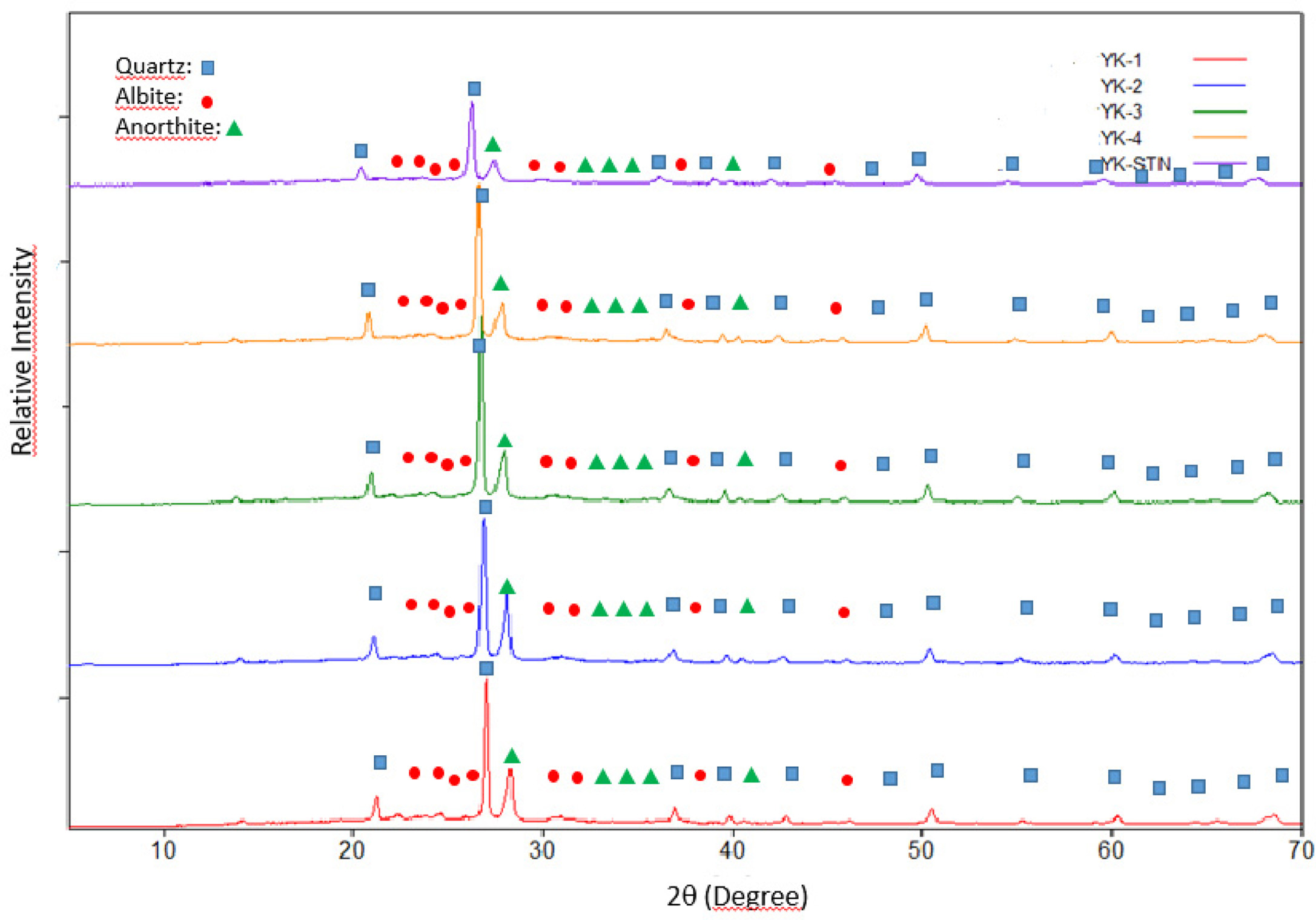
|
Fig. 2 Comparison of the XRD patterns of the floor tile compositions with standard floor tile body. |
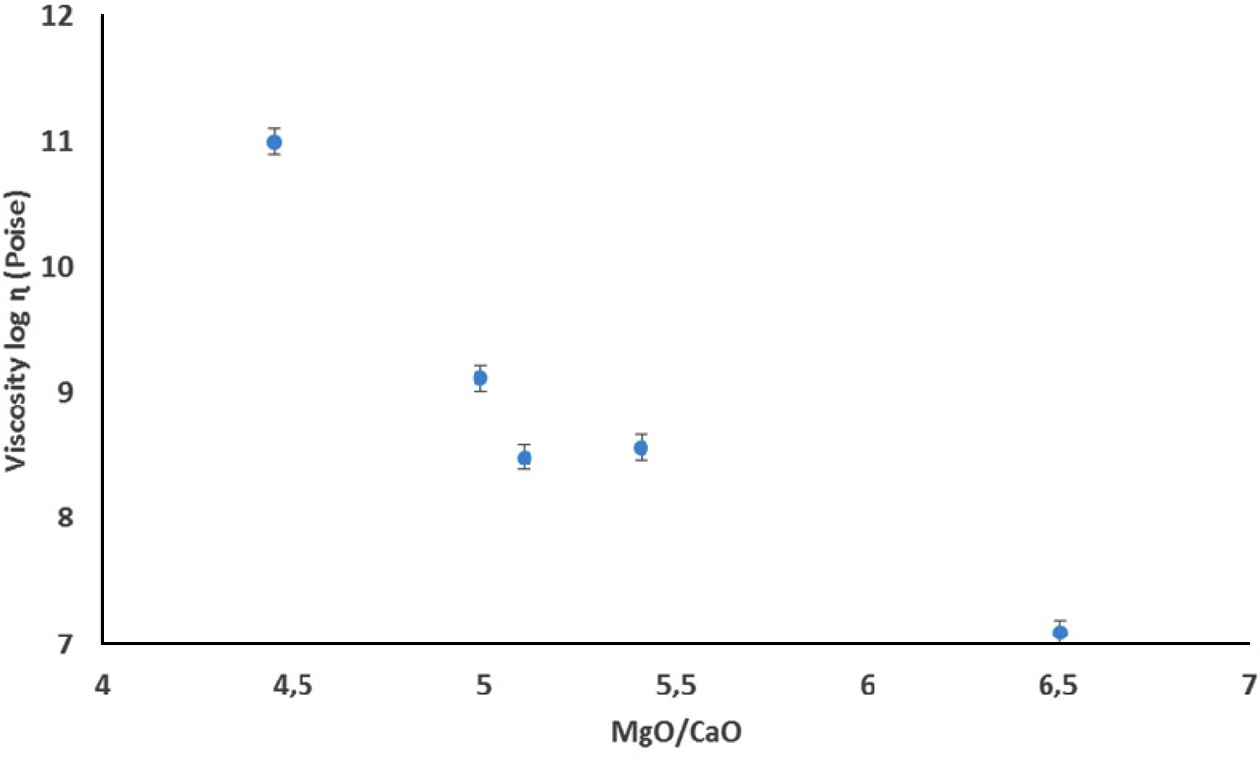
|
Fig. 3 Effect of MgO/CaO molar ratio on the viscosity of the floor
tile bodies at 1,160 oC. |
|
Fig. 4 Deformation behaviour of STD and new compositions. |
|
Fig. 5 SEM micrograph of the standard floor tile (STD). |
|
Fig. 6 SEM micrographs of the floor tile compositions. |
|
Fig. 7 EDX patterns taken from the standard floor tile (STD) compositon. (a) EDX analyses of irregular shaped crystal in STD composition,
(b) EDX analyses of a cluster of spheroidal crystals in STD composition. |
|
Fig. 8 EDX patterns taken from the newly developed porcelain stoneware tile (YK_2) composition. (a) EDX analyses of rounded shaped
crystal in (YK_2) composition, (b) EDX analyses of a irregular shaped crystals in (YK_2) composition. |
|
Fig. 9 EDX patterns taken from the newly developed porcelain stoneware tile (YK_3) composition. (a) EDX analyses of rounded shaped
crystal in (YK_3) composition, (b) EDX analyses of a irregular shaped crystals in (YK_3) composition. |
|
Table 3 Quantative mineralogical analyses of the floor tile
bodies by MAUD method after firing at 1,160 oC (in wt %). |
|
Table 4 Calculated viscosity values & Technological properties of the floor tiles |
In this study, floor tile compositions which contain
different MgO/CaO ratio, has been studied. It has been founded that MgO/CaO
ratio affects the viscosity of the floor tiles and as the ratio increases the
viscosity decreases. This viscosity decrease plays an important role for
pyroplastic behaviour of the tiles. This means that when effect of
compositional changes (in particular if there is any change in amount of liquid
phase formers) on sintering of the tiles are investigated, the viscosity should
be monitored and used as a key parameter during sintering of the tiles. For
YK_3 composition will be evaluated due to has allowable deformation amount for
the production. This floor tile composition will be successfully produce in
production line.
- 1. W.M. Carty and U. Serapati, J. Am. Ceram. Soc. 81[1] (1998) 3-20.
-

- 2. F.H. Norton, in “Fine ceramics: Technology and applica- tions” (McGraw Hill, 1970) p.70-75.
- 3. A. Dinsdale, in “Pottery science: Materials, Processes and Products” (Ellis Horwood Limited, 1986) p.40-52.
- 4. W.E. Warrol, in “Ceramic raw materials” (Pergamon, 1982) p.70-100.
-

- 5. A. Vari, in “Raw material preparation and forming of ceramic tiles” (S.A.L.A, 2000) p.45-60.
- 6. K.N. Maiti, C.S. Prasad, K.C. Singh, and A.K. Gupta, Trans. Ind. Ceram. Soc. 60[2] (2000) 77-81.
- 7. A.C. Bull, Trans. Brit. Ceram. Soc. 81[3] (1982) 69-74.
- 8. D.Y. Tuncel and E. Ozel, Ceram. Int. 38[2] (2012) 1399-1407.
-

- 9. Z.B. Ozturk and N. Ay, J. Ceram. Process. Res. 13[5] (2012) 635-640.
- 10. E. Rastelli, A. Tucci, L. Esposito, and L. Malmusi, Key Eng. Mater. 264-268 (2004) 1531-1534.
-

- 11. S. Elmas and İ. Tarhan, J. Sci. Perspect. 3[2] (2019) 85-98.
-

- 12. S.J.G. Sousa and J.N.F. Hollanda, Mater. Res. 8[2] (2005) 197-200.
-

- 13. E. Porte, R. Brydson, R. Rand, and R. Riley, J. Am. Ceram. Soc. 87[5] (2004) 923-928.
-

- 14. T. Aydın and A. Kara, J. Ceram. Process. Res. 15[6] (2014) 486-491.
- 15. M. Dondi, V. Biasini, G. Guarini, M. Raimondo, A. Argnani, and S. Primio, Key Eng. Mater. 206-213 (2002) 1795-1798.
-

- 16. T.K. Mukhopadghyay, M. Das, S. Glosh, S. Chacrabarti, and S. Ghatak, Ceram. Int. 29[5] (2003) 587-597.
-

- 17. V. Biasini, M. Dondi, G. Guarini, M. Raimondo, A. Argnani, and S.D. Primio, Silicates Industries 68[5-6] (2003) 67-73.
- 18. M. Paganelli, Am. Cer. Soc. Bull. 81[11] (2002) 25-30.
 This Article
This Article
-
2020; 21(6): 632-639
Published on Dec 31, 2020
- 10.36410/jcpr.2020.21.6.632
- Received on Mar 24, 2020
- Revised on Jun 9, 2020
- Accepted on Jun 10, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Neslihan Tamsu Selli
-
Department of Materials Science and Engineering, Gebze Technical University, 41400 Gebze, Kocaeli, Turkey
Tel : +902626052661 - E-mail: ntamsu@gtu.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.