- Application of bioelectromethanation using an electroactive methanogen for the biogas upgrading
Jisun Lee†, Jaesung Chun†, Okkyoung Choi* and Byoung-In Sang*
Department of Chemical Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
Bioelectromethanation was
tested using an isolated strain, Methanothermobacter sp., for biogas
upgrading. The investigated method showed faster bioelectrochemical conversion
of carbon dioxide to methane with higher coulombic efficiency than previously
reported without additional hydrogen and mediator supplementation.
Bioelectromethanation can utilize carbon dioxide, unlike gas separation
methods, and actually requires a less negative potential than in water
electrolysis. The isolated methanogens showed a relatively fast conversion to
methane compared to the previously reported methane production rate and current
intensity. Through further research on electroactive methanogens and the
development of operation technology, bioelectromethanation can be applied for
biogas upgrading with a lower energy requirement but without CO2
emissions.
Keywords: Bioelectrochemical conversion, Electrogenotrophic methanogen, Biogas upgrading, Methanation, CO2 utilization
Efforts to reduce carbon dioxide (CO2)
emissions are in progress worldwide in response to climate change issues.
Carbon capture and utilization (CCU) is an effective technology for reducing CO2
emissions. The reuse of CO2 would reduce the need for CO2 extraction
from natural sediment resources such as petroleum and enhance the circular CO2
economy [1]. Methane (CH4) is a reduced chemical for CO2 utilization
and can be used as a substitute for natural gases (95% methane, 5% ethane) to
generate electricity and make energy [2]. Highly concentrated methane can be
injected directly into a prevailing natural gas network to efficiently store energy
when needed and can stabilize renewable energy supply
systems with intermittent supply characteristics [3, 4]. Just as natural
gas is used as a vehicle fuel, a mixture with more than 95% methane can be used
as a transportation fuel [5-7]. In addition, methane is likely to be used as a
multipurpose chemical building block to make more value-added materials [8-11].
Biogas from anaerobic digestion contains approximately
40% CO2 and 60% CH4 [12]. Recently, biogas upgrading technology has attracted
attention for CO2 utilization and
waste-to-energy applications beyond the concept of gas purification, such as
absorption, adsorption, and membrane separation [13, 14]. The reduction of
CO2 requires reducing agents, generally hydrogen. To
convert CO2 to CH4, the amount of hydrogen
needed is 4 times the amount of CO2.
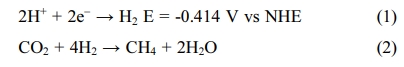
Electrical energy made from renewable energy, such as solar and wind, can
be converted into chemicals such as hydrogen
through water hydrolysis. The electrical
power equivalent of hydrogen (lower heating value) is 33.3 kWh/kg H2
[15]. Methanation eq. (2) can be carried out on both chemical and biological
catalysts. Hydrogenotrophic methanogens are used as a biological catalyst
[16-18].
Several
studies have suggested the bioelectromethanation (bioelectrochemical)
technique for biogas upgrading
[19-23]. The interaction between the microbes and cathode determines the product.
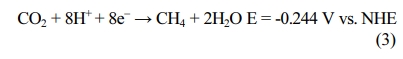
In electromethanogenesis, electrogenotrophic methano- gens can directly
use electrons from the cathode [22]. Bioelectromethanation is operated at a
less negative potential (-0.244 V), but the methane production is much lower
than those obtained with hydrogenotrophic methanogens.
This study used the recently isolated strain Methanothermobacter marburgensis, which is
strictly hydrogenotrophic
and thermophilic, to investigate its potential for ex situ
bioelectrochemical biogas upgrading. This study showed the bioelectrochemical
conversion from CO2 to CH4 by one isolated electroactive
methanogen, evaluated of pH effect on methane production, and suggested the
application of bioelectromethantion for biogas upgrading based on faster
conversion rate than previously reported.
Inoculums
The hydrogenotrophic and thermophilic methanogen denoted
as Methanothermobacter sp. THM-2 (NCBI taxonomy ID 2606912), which was
isolated from a thermophilic anaerobic digester and is phylogenetically related
to Methanothermobacter marburgensis DSM 2133T, was used as the inoculum.
The preculture was performed in a 1 L bottle at 60 oC with 200
mL ATCC medium containing (per liter) K2HPO4 2.04 g, KH2PO4
1 g, NH4Cl 1 g, NaCl 1 g, MgCl2∙6 H2O 0.1 g,
CaCl2∙2H2O 0.06 g, NaHCO3 4 g, resazurin (0.1%
w/v) 0.5 mL and trace elements 10 mL. The trace element solution contained (per
liter) nitrilotriacetic acid 12.8 g, FeSO4∙7H2O, MnSO4∙H2O
0.085 g, CaCl2∙2H2O 0.1 g, ZnCl2 0.1 g, H3BO3
0.01 g, NaCl 1 g, NiCl2∙6H2O 0.15 g, Al(SO4)2∙12H2O 0.098 g, CoCl2∙6H2O
0.024 g, CuCl2∙2H2O 0.025 g, Na2MoO4∙2H2O
0.024 g, NaSeO4 0.026 g, and Na2WO4∙2H2O
0.25 g. The medium was flushed with Ar gas for at least 30 min, and after
autoclaving at 121 oC for 20 min, Na2S∙9H2O
(0.5 g/L) was added. During the preculture, the headspace of the bottle was
exchanged with CO2/H2 (20/80) daily. The preculture was
operated for 3 weeks, vigorously mixing between gas-liquid at 500 rpm using
magnetic stirring.
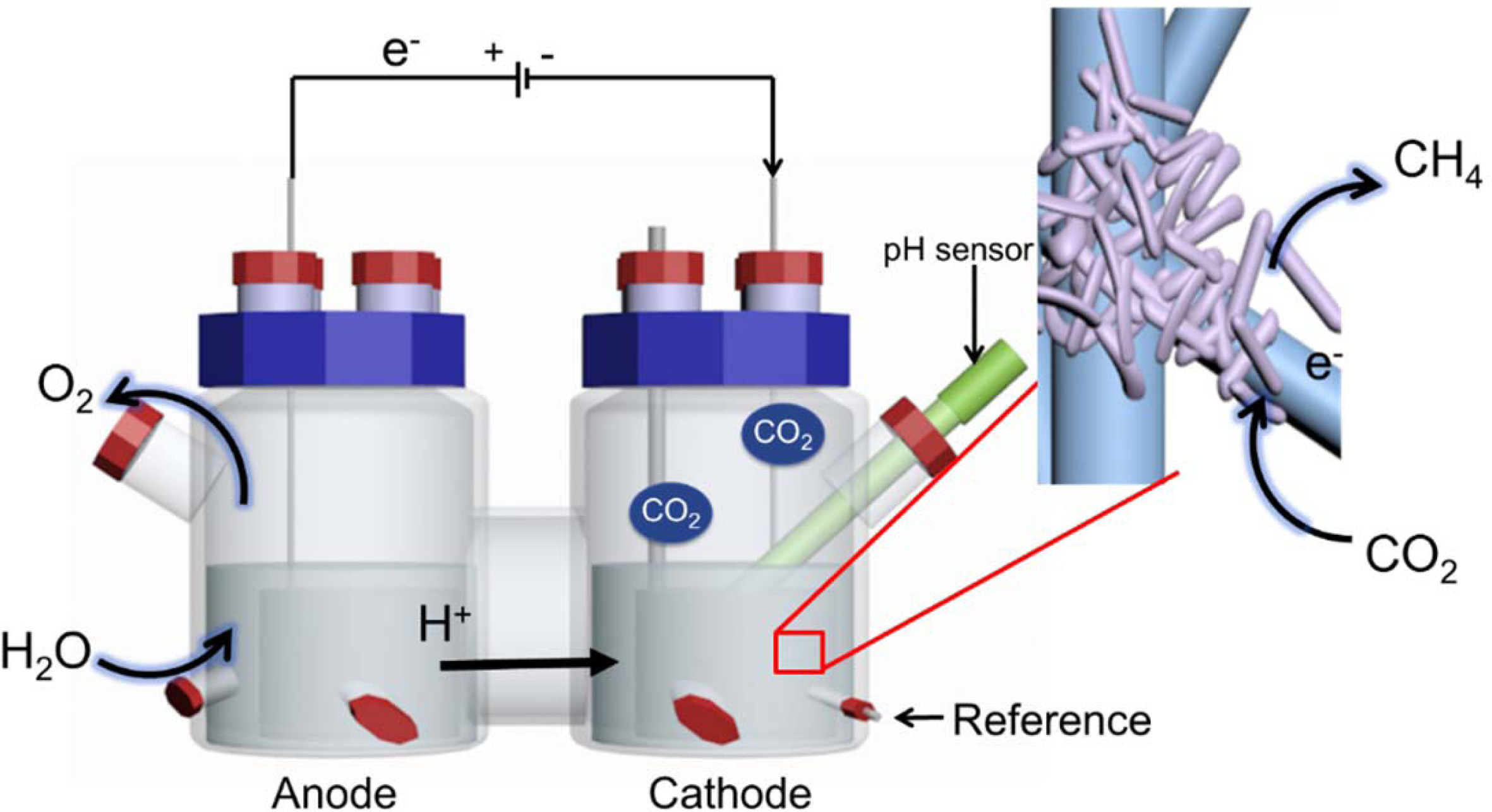
Bioelectrochemical
system (BES) start up and operation
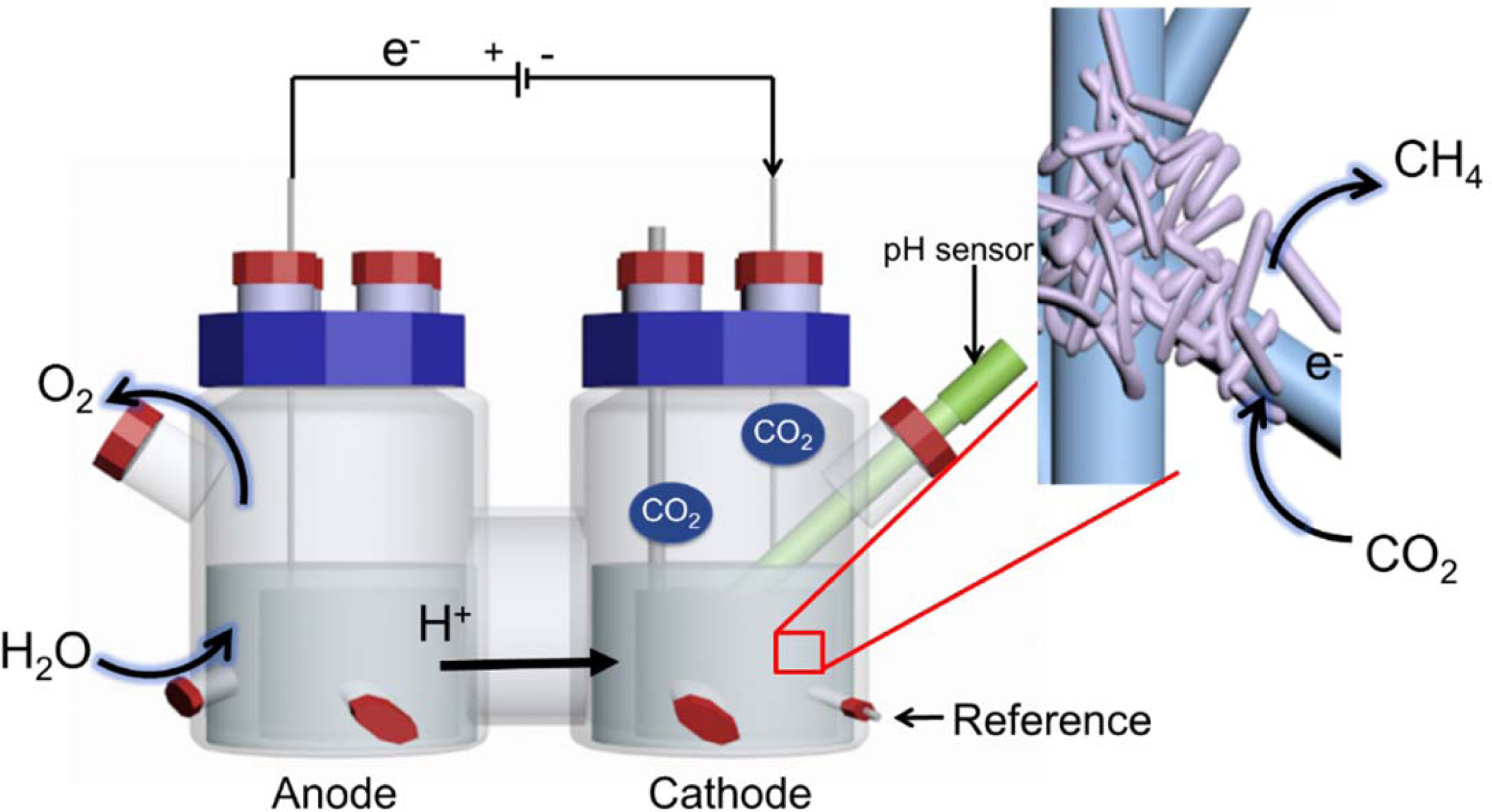
Graphite felt (6 cm×19.5 cm×0.5 cm) was used as the
cathode and anode and was inserted into the reactor in tubular form (area,
253.5 cm2). A three-electrode system (Fig. 1) was used, and a 3 M
KCl saturated Ag/AgCl electrode (+199 mV vs. SHE) was used as a reference
electrode. The electrode was attached to a titanium wire (1 mm in diameter)
using carbon paste and connected to a potentiostat (WMPG1000, South Korea). The
two-chamber reactor consisted of cathode and anode compartments and had a
4-port cap sealed with butyl rubber on the side. The cathode and anode were
separated by a proton exchange membrane (Nafionâ 117, Du Pont). A pH meter
(InLab Easy BNC, Mettler Toledo, USA) was inserted into the cathode
compartment. The volume of each chamber was 1.4 L, the headspace was 0.7 L, and
the working volume was 0.7 L. After assembly, the reactor with the electrode was
autoclaved at 121 oC for 20 min. After anaerobic treatment with
100% CO2 gas purging, 200 mL of Methanothermobacter sp. THM-2
cells were inoculated, and the initial optical density at 600 nm was
approximately 4. Gas exchange was carried out once a day with CO2.
To control the pH, 3.5 N H2SO4 and ammonia solution (28%
NH3 in H2O) were used. The experiment was performed at
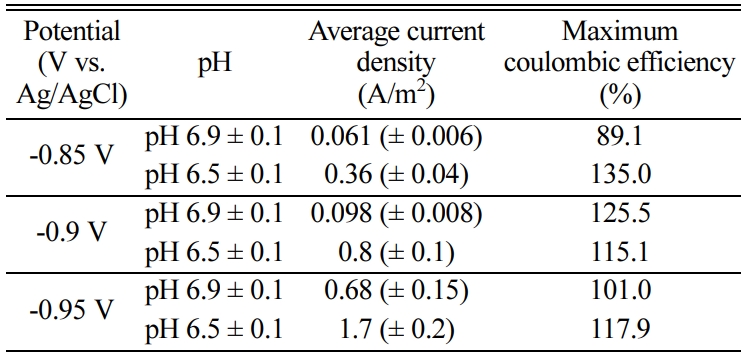
potentials of -0.85, -0.90 and -0.95 V vs
Ag/AgCl and different pH conditions (pH 6.9 ± 0.1, pH 6.5 ± 0.1).
Analysis
and calculations
The gas (CO2, H2, or CH4)
from the cathode was analyzed using GC-TCD (gas chromatography-thermal
conductivity detector, 7890A, Agilent, USA) with a Porapak Q Column (Supelco,
Inc, 6 ft ´ 1/8
in, SS, 80/100 mes), the oven temperature ranged from 40 to 80 oC
(10 oC/min), the injector temperature was 100 oC,
and the detector temperature was 200 oC.
The ammonium ion (NH4+) content was
measured using a RQflex 10 reflectometer (Merck, Germany) and a Reflectoquant®
Ammonium Test (Merck, Germany) kit. Coulombic efficiency was calculated using
the ratio of the electron number for the formation of the product to current
consumption.
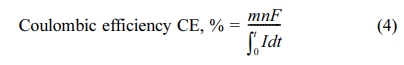
where m is the number of moles
of methane generated, n is the number of electrons required for the
formation of methane (8 eq./mol CH4, eq. (3)), F is the
Faraday constant (96,485 C/mol of electrons), and I is the circuit
current at time t.
|
Fig. 1 Bioelectromethanation reactor. The insert shows a schematic of the expected bioelectrochemical reaction at the interface of the
electrode and hydrogenotrophic methanogens. |
Bioelectrochemical
conversion of CO2 to CH4
After inoculation into the bioelectrochemical (BES)
reactor, the CO2 conversion to methane was detected. Under abiotic
conditions, methane production and current consumption were not
observed (data not shown). The methane production per unit
working volume was 0.05~0.23 L/L/d at pH 6.5 but 0.006~0.08 L/L/d at pH 6.9
and was enhanced at lower pH (Fig. 2). The
methane production per unit cathode surface
area was 6.36 L/m2/d at -0.95 V vs. Ag/AgCl and pH 6.5
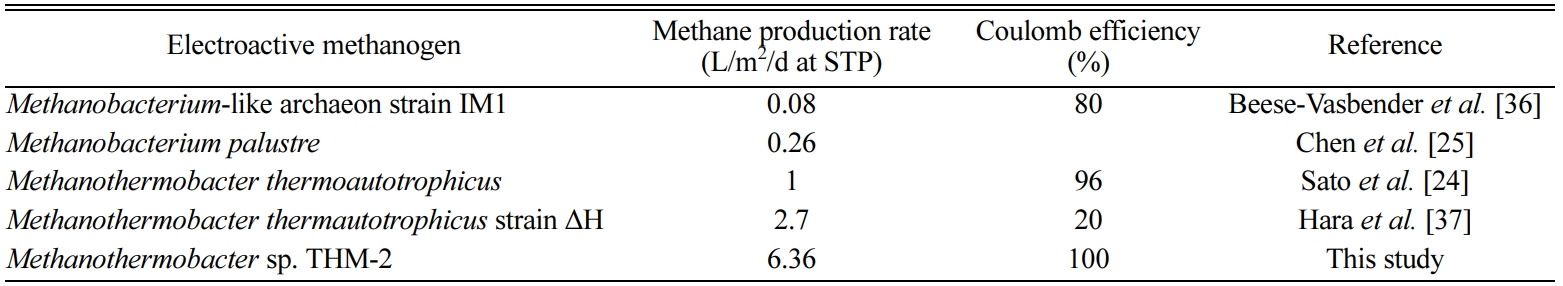
(Fig. 2). Studies on the bioelectrochemical conversion of CO2
reported methane production rates of 1 L/m2/d (273.15 K, 1 atm) in a pure
culture of Methanothermobacter thermoautotrophicus
[24] and 0.26 L/m2/d
with Methanobacterium palustre
[25] (Table 2). In general, pure methanogen culture applications of BES
resulted in lower reported methane production rates than mixed cultures, such
as anaerobic sludge, but this study showed a high methane production rate
similar to that of mixed cultures, reported as 1.5~9.8 L/m2/d [26].
The optical density (600 nm) was approximately 4 at the
initial time, and the value decreased slightly but did not change
significantly (data not shown). Methanogens are
sensitive against acidification, and the optimal pH range was previously
reported to be pH 6.5~8.0 [27]. The pH effect was tested at pH 6.5 vs. pH 6.9.
Interestingly, the bioelectrochemical conversion of CO2 to methane
was improved at pH 6.5 compared with that at pH 6.9 (Fig. 2 and Table 1). The
pH difference was small, but the methane production was significantly different.
Even at the same voltage, the bioelectrochemical conversion
of CO2 to methane was higher at pH 6.5 than at pH
6.9 (Fig. 2). There have been no studies about the
pH effect on BES in pure cultures of hydrogenotrophic methanogens.
The reason why methane production increased at lower pH is still
unknown, but it is speculated that electron
transfer between the electrode and methanogens is more active at
pH 6.5 than at pH 6.9. As a follow-up study, we plan to further characterize the
isolated electrotrophic methanogen strain to determine why these
results were observed and develop operating conditions for increased methane
production.
Coulombic
efficiency
In
addition, the average current density was 0.06~1.7 A/m2 (Table 1). This current density was also
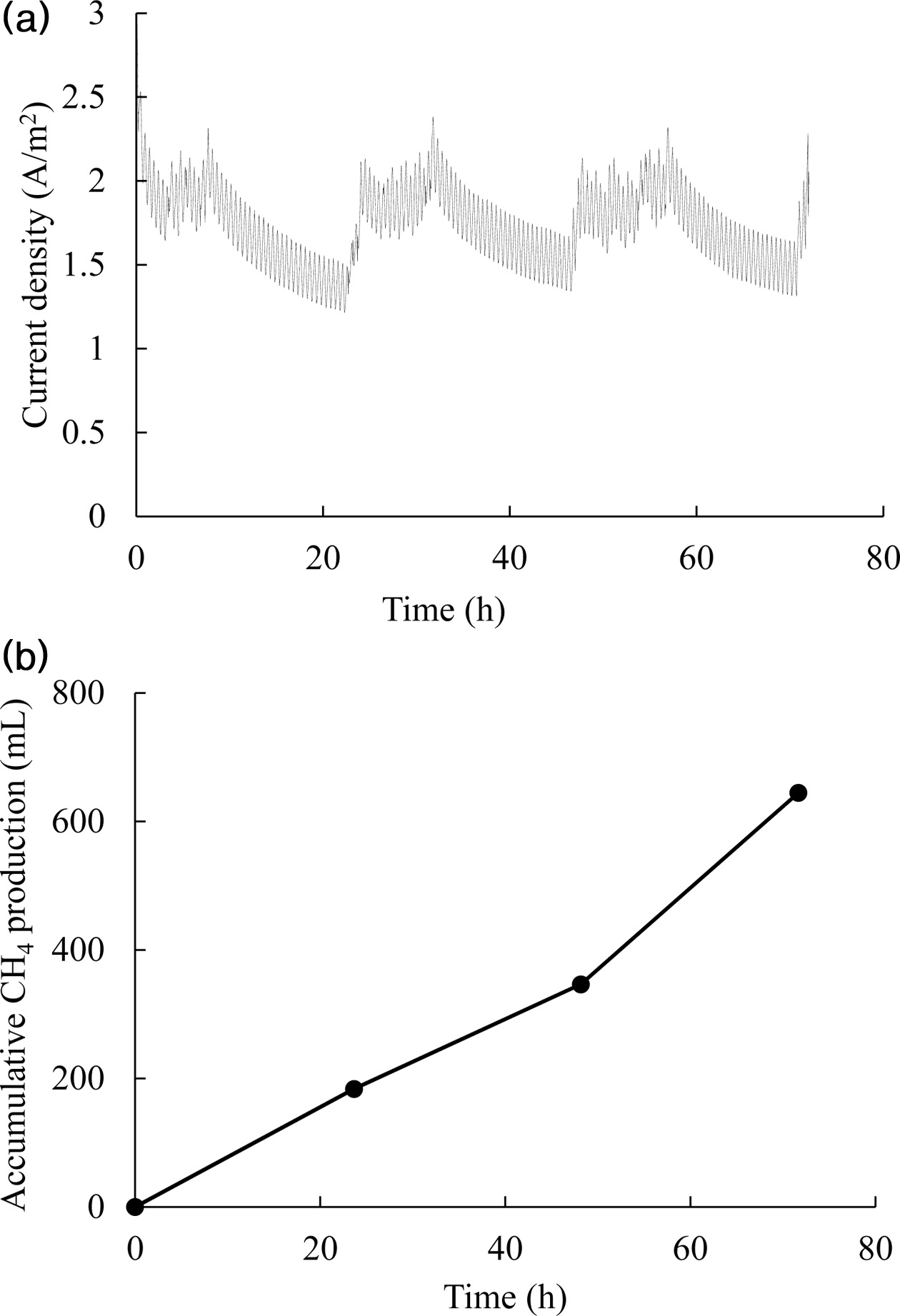
higher than that previously reported for pure culture methanogens, < 0.5 A/m2 [28]. Fig. 3 shows the current
density and methane accumulation over time at -0.95 V vs Ag/AgCl and pH 6.5. After CO2 injection,
the current increased rapidly but
decreased with CO2 consumption. Under all conditions, the amount of
methane produced was found to be
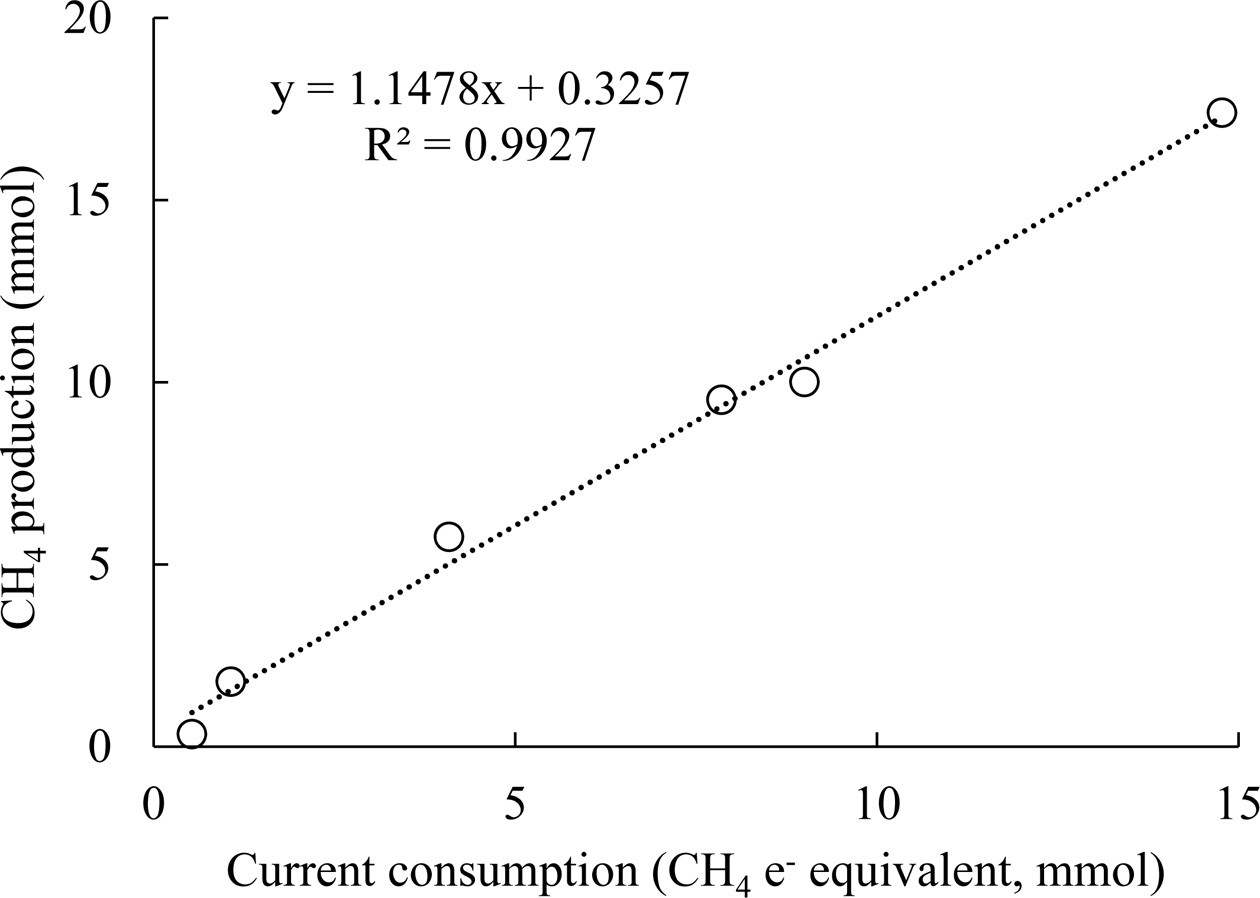
linearly related to current consumption,
and the ratio of approximately 1 in Fig. 4 indicates nearly 100% coulombic efficiency (eq. (4)). High
methane production and current
density increased the coulombic efficiency to near 100%; the slope value is 1.1
in Fig. 4. According to past research data, the reported highest level of coulombic efficiency for methane, CH4,
is approximately 64% when using a
copper single-crystal catalyst [29].
The abiotic electrochemical reduction of CO2 exhibits a lower energy efficiency on metal electrodes
[30]. In a bioelectrochemical system, the reported coulombic efficiencies of a
mixed culture and pure culture were
82.6±15.7% and 55±27%, respectively,
and the mixed cathodic culture showed higher methane production [26]. However,
in the case of microbial fuel cells that were studied much earlier, a pure
inoculum with defined electroactive microorganisms showed higher efficiency [31]. Our study suggests the
possibility of better efficiency when
using a pure culture than mixed
cultures in the cathode after operational parameter development.
In general, the higher the voltage is, the higher the
methane production [26]. A more negative potential increases the hydrogen
production when converting CO2 to CH4, i.e., indirect
electron transfer occurs using H2 as an electron carrier. At -0.95 V
vs. Ag/AgCl, the amount of hydrogen detected was only ~5.5% of the CH4
amount. Because 4 H2 molecules are needed for 1 CH4
molecule, the electron number required for 1 CH4 generation is the
same in both direct (8 e-)
and indirect electron transfer (4 ´ 2 e- = 8 e-). Additionally, the current
consumption required for methane production is the same if both the direct
(electron) and indirect (H2) processes have the same efficiency. The
observed high coulombic efficiency at -0.95 V vs. Ag/AgCl indicates efficient
electron transfer at the given potential.
Application
of bioelectrochemical conversion for biogas upgrading
This study shows the possibility of applying the
bioelectrochemical conversion of CO2 to CH4 using an electroactive
methanogen for biogas upgrading. Despite having
the advantage of operating at less negative voltages (eq.
(1) vs. eq. (3)), the slow methane production rate and low
current densities of the bioelectrochemical system [19, 28] are the main
reasons making this system unsuitable for real methanation applications.
Nevertheless, the methane production rate is much lower than that of
biomethanation using thermophilic hydrogenotrophic methanogens. The highest
methane production rate reported was 288 L/L/d under atmospheric pressure
[17]. Bioelectrochemical conversion occurs at the surface
of the electrode, and the methane production rate is
indicated per unit surface area, not volume. Therefore, it is difficult to
directly compare bioelectro- methanation with
biomethanation. In the bioelectro-
methanation approach in this study,
the rate of methane production per electrode surface was 6.4 (±0.8) L/m2/d,
and the rate per working volume was
0.23 (±0.03) L/L/d at -0.95 V vs.
Ag/AgCl and pH 6.5. A low conversion rate is difficult to apply to processes
with a high gas hourly space velocity (GHSV) treating a large amount of CO2.
The typical biogas generation rate is 0.3-0.5 L/L/d, and biogas contains
approximately 40% CO2 [32]. Based on previous data, the amount of CO2
treated for conversion to CH4 is 0.1-0.2 L/L/d.
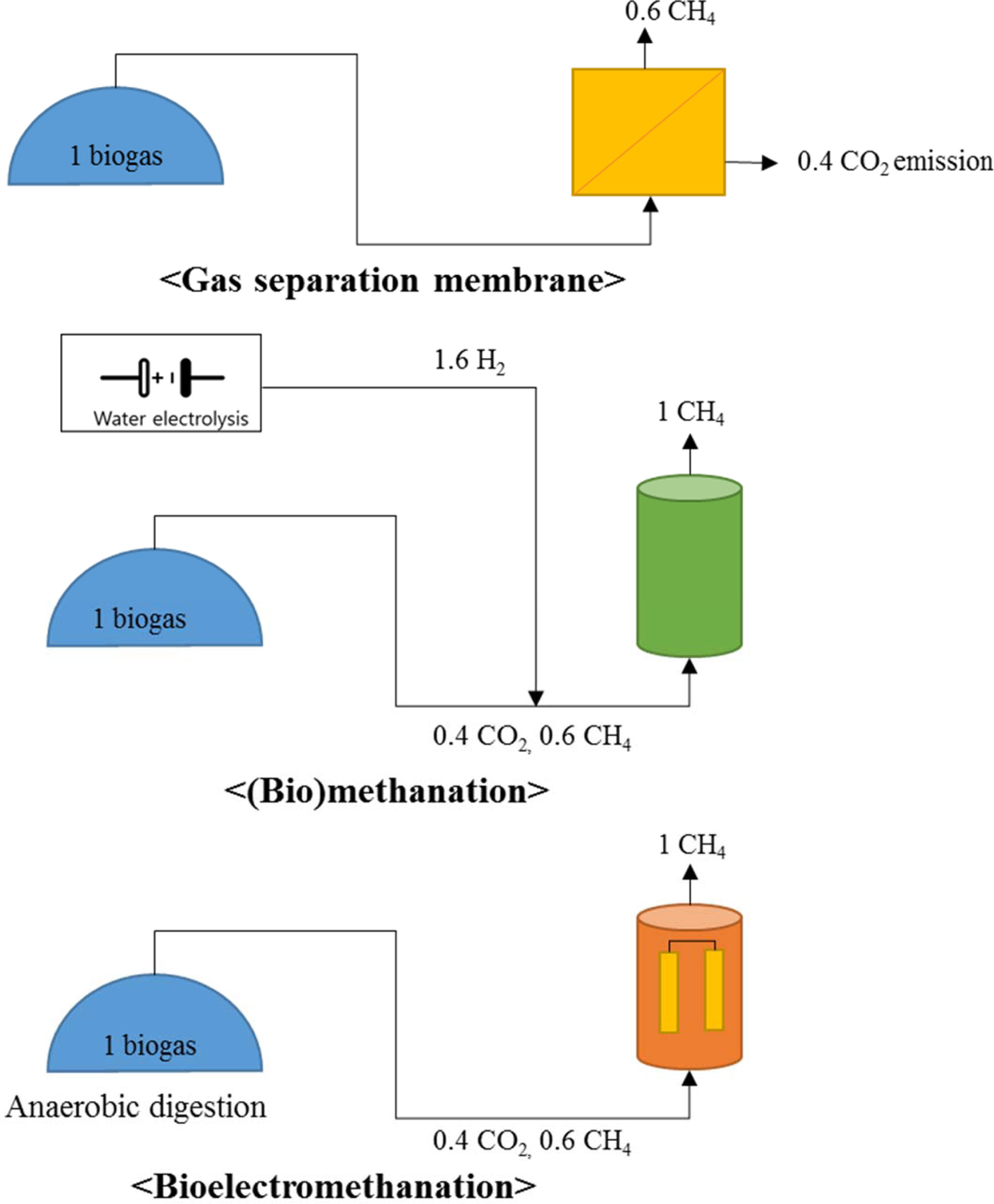
Therefore, we propose a biogas system combined with bioelectro- methanation
for biogas upgrading that does not require a fast conversion rate (Fig. 5).
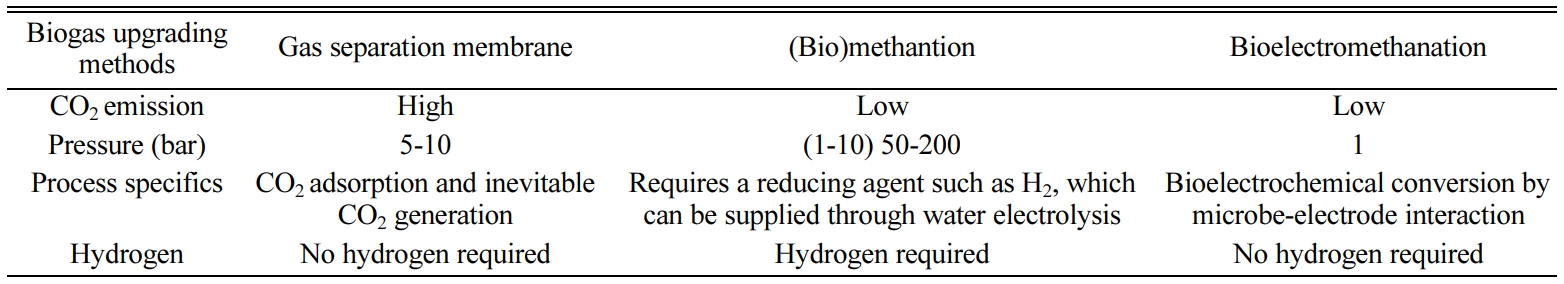
Table 3 shows charac- teristics of
three biogas upgrading technology. Biogas upgrading using a gas separation
system emits CO2 and produces a relatively small amount of methane
(Fig. 5(a)). The theoretical external voltage of the cathode for bioelectromethanation
(eq. (3)) is less than that for hydrogen production (eq. (1) and Fig. 5(b)) in
water electrolysis [21, 24]. Additionally,
there is no need for an additional reactor that produces a reducing agent, such
as H2 (Fig. 4(b)), because electricity supply and conversion take
place in one reactor (Fig. 5(c)). The substrate for bioelectromethanation is
only CO2, and a pure
culture can prevent competition between methanogens (-0.24 V vs. Ag/AgCl) and acetogens (-0.29 V vs.
Ag/AgCl) [33, 34]. Because autotrophic methanogens are used, no organic carbon
is required, and only ammonia (nitrogen source), sulfide (enzyme precursor),
and trace metal (enzyme cofactor) are needed for bioelectromethanation. Therefore, bioelectromethanation is relatively easy to apply as a module in anaerobic digesters
(Fig. 5(c)). The bioelectromethantion can be simply installed as a module to increase methane concentration of
biogas. Based on our conversion rate,
bioelectromethanation can be applied for biogas upgrading (~0.02 GHSV, gas
hourly space velocity) requiring lower GHSV than biological methanation (~100 GHSV) or chemical methanation (500~5,000 GHSV) [35].
|
Fig. 2 pH-dependent methane production in the bioelectrochemical system. |
|
Fig. 3 Current density (a) and cumulative methane production (b)
obtained when operating the BES at -0.95 V vs. Ag/AgCl and pH
6.5. CO2 charging into the bioelectrochemical reactor was
performed three times at the times of current decrease. |
|
Fig. 4 Methane production with respect to current consumption.
The current consumption is indicated in methane electron
equivalents, i.e., the number of moles of electrons divided by 8 (eq.
(2)). |
|
Fig. 5 A comparison of gas separation, biochemical methanation and bioelectromethanation as biogas upgrading methods.
Bioelectromethanation can convert CO2 to CH4 directly using electricity |
|
Table 2 The comparison of methane production rate and coulomb efficiency reported previously |
This study showed fast conversion rate of CO2
to CH4 by pure methanogen-electrode reaction, up to the level
applicable to actual process and it represented that it can be applied to
actual processes such as biogas upgrading system.
Bioelectromethanation has the advantage of not requiring expensive
hydrogen, but it has been reported that the reaction rate and efficiency was
low for practical application. However, our results suggest that a pure
electrogenotrophic methanogen can be used for bioelectromethanation, especially
biogas upgrading, with relatively faster conversion rate than previously
reported value. This approach allows for sustainable
energy systems in anaerobic digestion plants, and
the environmental incentives are expected to evolve.
This work was supported in part by a grant funded by
Hanyang University in the Republic of Korea (HY-201100000000233-N) and by the
Korea Institute of Energy Technology Evaluation and Planning (KETEP) and
the Ministry of Trade, Industry & Energy (MOTIE) of the
Republic of Korea (No. 20171520101740 & 2019281010007B).
The authors declare no conflict of interest.
- 1. M. Peters, B. Köhler, W. Kuckshinrichs, W. Leitner, P. Markewitz, and T.E. Müller, Chem. Sus. Chem. 4[9] (2011) 1216-1240.
-

- 2. B. Viswanathan, in “Energy Sources” (Elsevier, 2017) 59-79.
-

- 3. H. Blanco, W. Nijs, J. Ruf, and A. Faaij, Appl. Energy 232 (2018) 323-340.
-

- 4. M. Prussi, M. Padella, M. Conton, E.D. Postma, and L. Lonza, J. Cleaner Prod. 222 (2019) 565-572.
-

- 5. G. Senthamaraikkannan, D. Chakrabarti, and V. Prasad, in “Future Energy” (Elsevier, 2014) 271-288.
-

- 6. V. Makareviciene, E. Sendzikiene, S. Pukalskas, A. Rimkus, and R. Vegneris, Energy Conversion and Management 75 (2013) 224-233.
-

- 7. U. Kesieme, K. Pazouki, A. Murphy, and A. Chrysanthou, Sustainable Energy & Fuels 3[4] (2019) 899-909.
-

- 8. K.T. Smith, S. Berritt, M. González-Moreiras, S. Ahn, M.R. Smith, M.-H. Baik, and D.J. Mindiola, Science 351[6280] (2016) 1424-1427.
-

- 9. E. de Jong, A. Higson, P. Walsh, and M.J.I.B. Wellisch, Task42 Biorefinery 34 (2012).
- 10. S.R. Hughes, W.R. Gibbons, B.R. Moser, and J.O. Rich, in “Biofuels: Economy, Environment and Sustainability” (InTech, 2013) 245-267.
- 11. E. de Jong and G. Jungmeier, in “Industrial Biorefineries & White Biotechnology” (Elsevier, 2015) 3-33.
-

- 12. A.I. Adnan, M.Y. Ong, S. Nomanbhay, K.W. Chew, and P.L. Show, Bioengineering 6[4] (2019) 92.
-

- 13. A. Al-Mamoori, A. Krishnamurthy, A.A. Rownaghi, and F. Rezaei, Energy Technol. 5[6] (2017) 834-849.
-

- 14. I. Angelidaki, L. Treu, P. Tsapekos, G. Luo, S. Campanaro, H. Wenzel, and P. Kougias, Biotechnol. Adv. 36[2] (2018) 452-466.
-

- 15. D. Gielen, in “Hydrogen from renewable power technology outlook for the energy transition” (IRENA, 2018) 50.
- 16. O. Choi, M. Kim, Y. Go, M.-G. Hong, B. Kim, Y. Shin, S. Lee, Y.G. Kim, J.S. Joo, B.S. Jeon, and B.-I. Sang, Energies 12[21] (2019) 4130.
-

- 17. J.-P. Peillex, M.-L. Fardeau, and J.-P. Belaich, Biomass 21[4] (1990) 315-321.
-

- 18. D. Rusmanis, R. O’Shea, D.M. Wall, and J.D. Murphy, Bioengineered 10[1] (2019) 604-634.
-

- 19. F. Geppert, D. Liu, M. van Eerten-Jansen, E. Weidner, C. Buisman, and A. ter Heijne, Trends Biotechnol. 34[11] (2016) 879-894.
-

- 20. X. Jin, Y. Zhang, X. Li, N. Zhao, and I. Angelidaki, Environ. Sci. Technol. 51[16] (2017) 9371-9378.
-

- 21. A.B.T. Nelabhotla, and C. Dinamarca, Appl. Sci. 9[6] (2019) 1056.
-

- 22. H. Xu, K. Wang, and D.E. Holmes, Bioresour. Technol. 173 (2014) 392-398.
-

- 23. L. Zhang, A. Kuroki, and Y.W. Tong, Front. Energy Res. 8 (2020).
-

- 24. K. Sato, H. Kawaguchi, and H. Kobayashi, Energy Convers. Manage. 66 (2013) 343-350.
-

- 25. S. Cheng, D. Xing, D.F. Call, and B.E. Logan, Environ. Sci. Technol. 43[10] (2009) 3953-3958.
-

- 26. Y. Jiang, H.D. May, L. Lu, P. Liang, X. Huang, and Z.J. Ren, Water Res. 149 (2019) 42-55.
-

- 27. K. Anderson, P. Sallis, and S. Uyanik, in “Handbook of Water and Wastewater Microbiology” (Academic Press, 2003) 391-426.
-

- 28. B.E. Logan, R. Rossi, A.A. Ragab, and P.E. Saikaly, Nat. Rev. Microbiol. 17[5] (2019) 307-319.
-

- 29. N. Ali, M. Bilal, M.S. Nazir, A. Khan, F. Ali, and H.M.N. Iqbal, Sci. Total Environ. 712 (2020) 136482.
-

- 30. Y.I. Hori, in “Modern aspects of electrochemistry” (Springer, 2008) 89.
-

- 31. X. Zhang, X. Li, X. Zhao, and Y. Li, RSC Adv. 9[34] (2019) 19748-19761.
-

- 32. U. Werner, U. Stöhr, and N. Hees, Deutsches Zentrum für Entwicklungstechnologien-GATE (1989).
- 33. S.D. Molenaar, P. Saha, A.R. Mol, T.H.J.A. Sleutels, A. Ter Heijne, and C.J.N. Buisman, Int. J. Mol. Sci. 18[1] (2017) 204.
-

- 34. J. Philips, Front. Microbiol. 10 (2020) 2997.
-

- 35. M. Götz, A. Koch, and F. Graf, in Proceedings of the International Gas Union Research Conference (IGRC), Setemper 2014, edited by J. Lewnard (Danish Gas Technology Center, 2014) p.TO5-4.
- 36. P.F. Beese-Vasbender, J.-P. Grote, J. Garrelfs, M. Stratmann, and K.J.J. Mayrhofer, Bioelectrochemistry 102 (2015) 50-55.
-

- 37. M. Hara, Y. Onaka, H. Kobayashi, Q. Fu, H. Kawaguchi, J. Vilcaez, and K. Sato, Energy Procedia 37 (2013) 7021-7028.
-

 This Article
This Article
-
2020; 21(5): 602-608
Published on Oct 31, 2020
- 10.36410/jcpr.2020.21.5.602
- Received on Jun 12, 2020
- Revised on Jul 14, 2020
- Accepted on Aug 14, 2020
 Services
Services
- Abstract
introduction
methods and materials
results and discussion
conclusion
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Okkyoung Choi and Byoung-In Sang
-
Department of Chemical Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
Tel: +82-2-2220-4717, Fax: +82-2-2220-4716 (Okkyoung Choi)
Tel: +82-2-2220-2328, Fax: +82-2-2220-4716 (Byoung-In Sang) - E-mail: okgii77@hanyang.ac.kr, biosang@hanyang.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.