- Zirconium doped LiNi0.91Co0.06Mn0.03O2cathode as a superior cathode for lithium ion batteries
Seung-Hwan Lee*
Department of Advanced Materials Engineering, Daejeon University, Daejeon 34520, Republic of Korea
In this paper,
well-crystallized Zr-doped Ni-rich layered LiNi0.91Co0.06Mn0.03O2
cathode is prepared by solid state method and its structural properties and
electrochemical performances in lithium-ion cells is investigated. The Zr-doped
LiNi0.91Co0.06Mn0.03O2 has superior cation ordering
and the morphology of Zr-doped LiNi0.91Co0.06Mn0.03O2
was the same as pristine LiNi0.91Co0.06Mn0.03O2. The 0.5 wt% Zr-doped LiNi0.91Co0.06Mn0.03O2
shows better electrochemical performances such as initial discharge
capacity of 210.5 mAh g-1, rate capability of 75.4 % at 2 C and
cycling retention of 94.5 % after 50 cycles. It can be elucidated by the role
of the Li2ZrO3 layer and Zr substitution in LiNi0.91Co0.06Mn0.03O2.
Keywords: Zr-doped Ni-rich layered LiNi0.91Co0.06Mn0.03O2, Cation ordering, Morphology, Electrochemical performances
Nowadays, lithium ion batteries (LIBs) have received
attention as an outstanding main power source to meet the high energy density
for electric vehicles (EVs) and portable devices. It is well known that cathode
is the key component, determining the battery performance [1, 2].
Among many cathodes, the Ni-rich LiNixCoyMnzO2
(NCM, x ≥ 0.8, x + y + z = 1) materials
have stood out as the promising candidate due to its smaller volume change
(LiCoO2), superior specific capacity (LiNiO2) and
excellent thermal stability (LiMn2O4) [3]. Unfortunately,
Ni-rich NCM cathodes still suffer from inferior long-term
cycling performance, especially, it is proportional to the Ni content. It can be
explained by unwanted side reaction at the interface between
cathode and electrolyte. It leads to phase transformation from
layered to disordered spinel/rock-salt, the reduction of Ni4+ and
oxygen loss, resulting in severe structural degradation [4]. This phenomenon
causes the rapid increase of cathode impedance and capacity decay of Ni-rich
NCM during cycling [5].
Many approaches such as coating [6], doping [7] and single
crystal [8] have been studied to enhance the electrochemical performance of
Ni-rich NCM. Among them, a lot of doping effects have been reported to increase
cycle life of Ni-rich cathodes [9-11]. This is because the dopant ion can
reduce the cation mixing, stabilizing the crystal structure from volume changes
during cycling. Among various dopants, the Zr-doped NCM cathode showed
excellent electrochemical performance. However, as far as we know, there have been no reports of NCM
cathodes with a Ni content of 91%.
In this paper, we synthesize the Zr-doped Ni-rich LiNi0.91Co0.06Mn0.03O2
(NCM91) with high crystallinity and investigate the effect of doping to improve
the structural stability and electrochemical performances. These
results indicate that suitable amount of Zr substitution delivers the superior electrochemical
performances.
Ni0.91Co0.06Mn0.03(OH)2
precursor was prepared by using aqueous solution of NiSO4·6H2O,
CoSO4·7H2O and MnSO4·H2O via a
co-precipitation method. A NaOH and NH4OH solution as a chelating
agent were used. The as-prepared Ni0.91Co0.06Mn0.03(OH)2
precursor was mixed with LiOH·H2O at a molar ratio of 1 :
1.05 and 0.5 wt% ZrO2 as a zirconium source in a molar ratio of 1.0
wt%. Afterward, the mixed powders were sintered at 500 oC for 5
h and 680 oC for 15 h in air for pristine and Zr-doped
NCM91.
The structural properties of the pristine and Zr-doped
NCM91 were conducted by X-ray diffraction (XRD, X-pert PRO MPD, Philips, Cu Kα)
and field emission scanning electron microscopy (FESEM, S-4800, HITACHI) equipped with an energy dispersive X-ray
detector (EDX, X-maxN, HORIBA).
All electrochemical performances were evaluated based on a
2032 coin cells. The pristine and Zr-doped NCM91 cathodes were prepared by
mixing active materials, super P black (conductive material) and polyvinylidene
fluoride (PVDF, binder) with a weight ratio of 96 : 2 : 2. After that, the
mixed slurry was coated on Al foil (16 μm in thickness) and dried at 120 oC
for 12 h in a vacuum oven. The prepared cathode was punched with an electrode
diameter of 14 mm and then dried at 120 oC for 12 h in a vacuum
oven. 1M LiPF6 in ethylene carbonate / ethylmethyl carbonate / dimethyl
carbonate (1:1:1, v/v/v) was adopted as an electrolyte. Finally, coin cells
were fabricated in an argon-filled glove box.
The electrochemical test was carried out galvanostatically in the voltage range of
3.0-4.3 V and various C-rates (1C = 210 mAh g-1)
using electrochemical equipment (TOSCAT-3100, Toyo system) at room temperature.
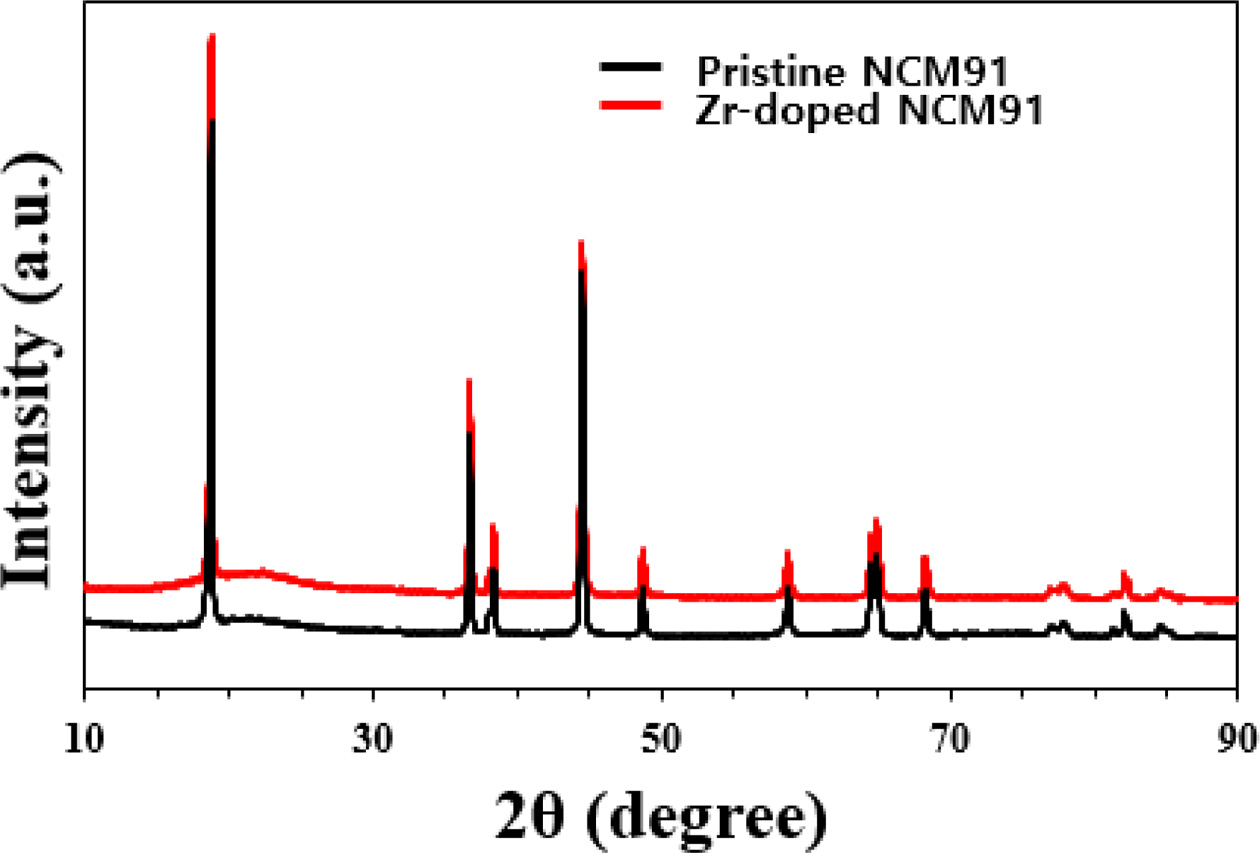
Fig. 1 shows the XRD patterns of pristine and Zr-doped
NCM91. All the samples exhibited almost similar XRD patterns since small amount
Zr doping does not significantly affect the NCM91 structure. All patterns
indicate that synthesized samples belong to a layered hexagonal α-NaFeO2
structure with the space group R-3m [2, 5]. The clear peak splitting of
(006)/(102) and (108)/(110) indicates the well-developed hexagonal layered
structure. However, for Zr-doped NCM91, there is a slight shift in the peak
position to lower angles compared to the pristine NCM91. It can be explained by
bigger ionic radius of Zr (0.72 Å) than that of Ni2+ (0.69 Å).
Therefore it can be confirm that Zr ions diffuse into the NCM91 structure. As
listed in Table 1, the I(003)/I(104) ratios of the
Zr-doped NCM91 are greater than that of pristine NCM91, indicating better
structure stability and lower cation mixing of Zr-doped NCM91. It is believed
that the Zr-doped NCM cathode can achieve high performances based on excellent
structural stability.
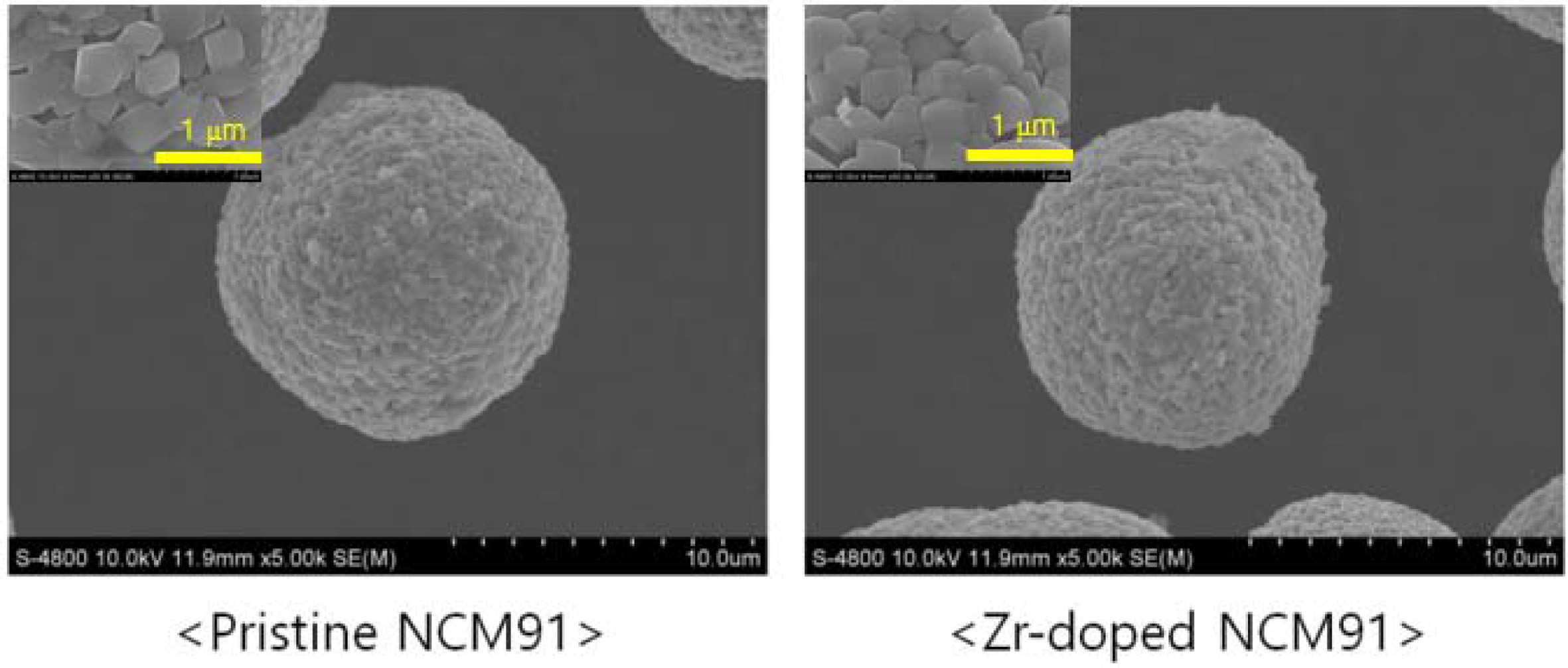
Fig. 2 shows the FESEM images of the pristine and Zr-doped
NCM91. All samples have similar spherical morphology with an average size of 12
μm. Also, the spherical secondary particles are composed of small primary
particles of approximately 200-500 nm. It can be seen that Zr doping not only
does not change the spherical shape of NCM91, but also has little effect
on the particle size. Moreover, the primary particles contacted
each other directly. This can lower the resistance
and improve the electrochemical performances. More
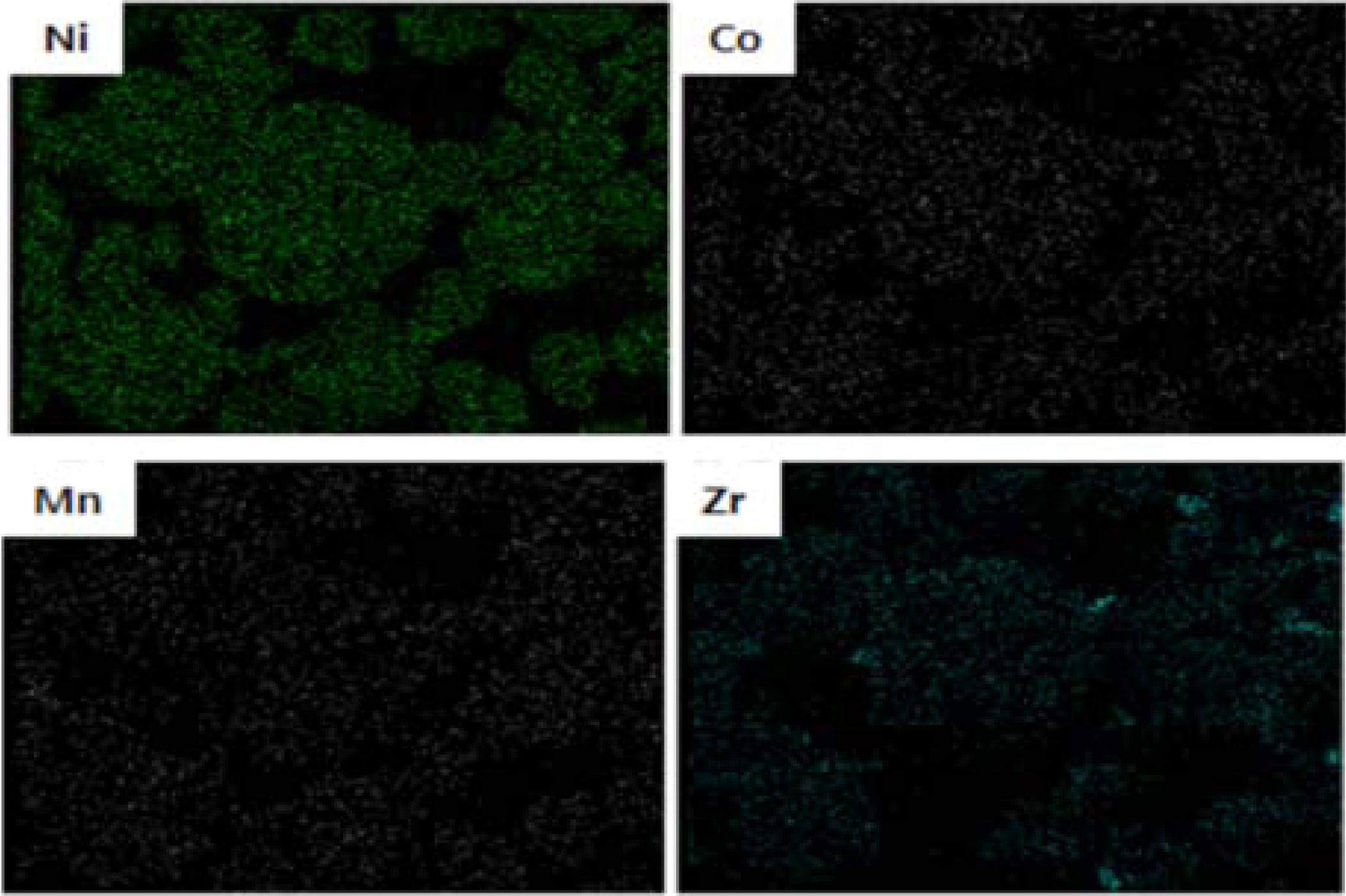
importantly, we can see that the elements of Ni, Co, Mn and Zr were uniformly
distributed, as shown in Fig. 3. We believe that the uniformly Zr-doped NCM91 can
maximize the structural stability and electrochemical
performances.
For electrochemical tests, the loading level of the NCM91
was adjusted about 14.2 mg/cm2 because the high areal capacity is
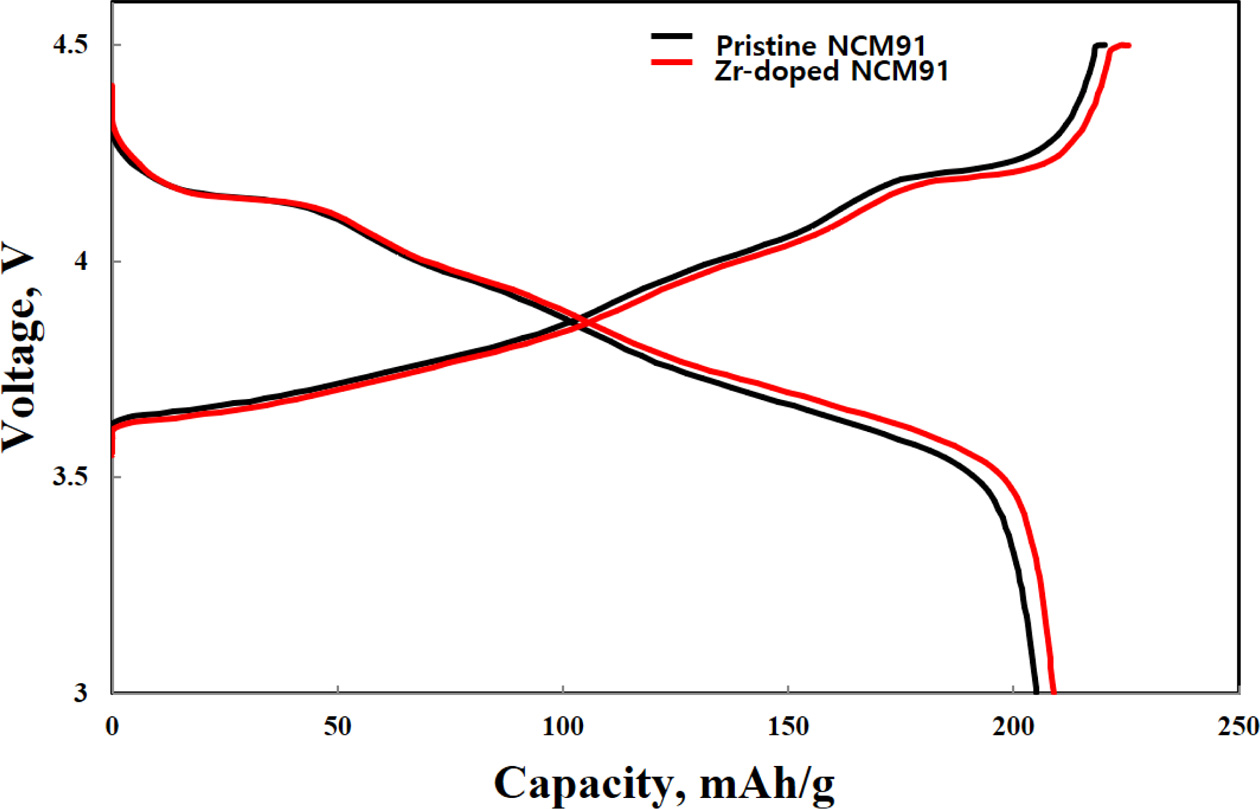
essential for practical application of LIBs [3]. Fig. 4 delivers the initial
charge-discharge curves with pristine and different amount of Zr-doped NCM91 in
a potential range of 3.0-4.3 V at 0.1 C. All samples have similar
charge-discharge behaviors. It indicates that the Zr doping does not influence
on electrochemical behaviors of the NCM91. The pristine NCM91 delivers the
lower discharge capacity (216.8 mAh g-1).
However, Zr-doped NCM91 shows higher discharge curves (210.5 mAh g-1),
originated from the Zr incorporated into the crystal lattice. It can be
explained by the enhanced conductivity of NCM91 compared to that of pristine
NCM91 [5].
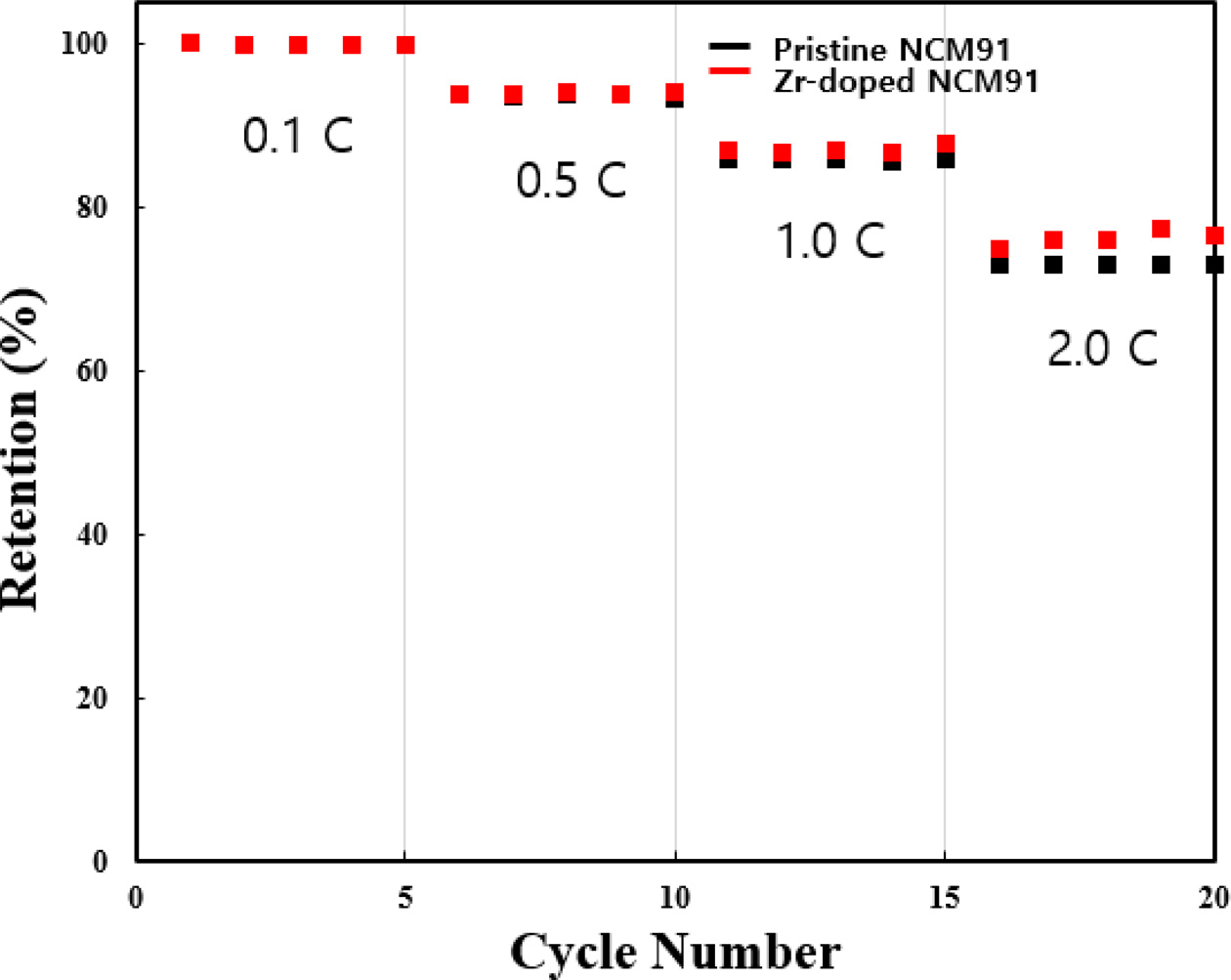
The rate capability of pristine and Zr-doped NCM91 at
various C-rates from 0.1 C to 2 C are shown in Fig. 5. We can confirm that the
capacity retention of all samples decreases as the C-rate increases. At low
C-rates, all samples have almost the same capacity retention. However, as C-rate
increased, the retention of pristine NCM91 sharply decreases, whereas Zr-doped
NCM91 can suppress the retention decrease. It is closely related to the lower
resistance of Zr-doped NCM91, resulting in fast Li ion kinetics [8].
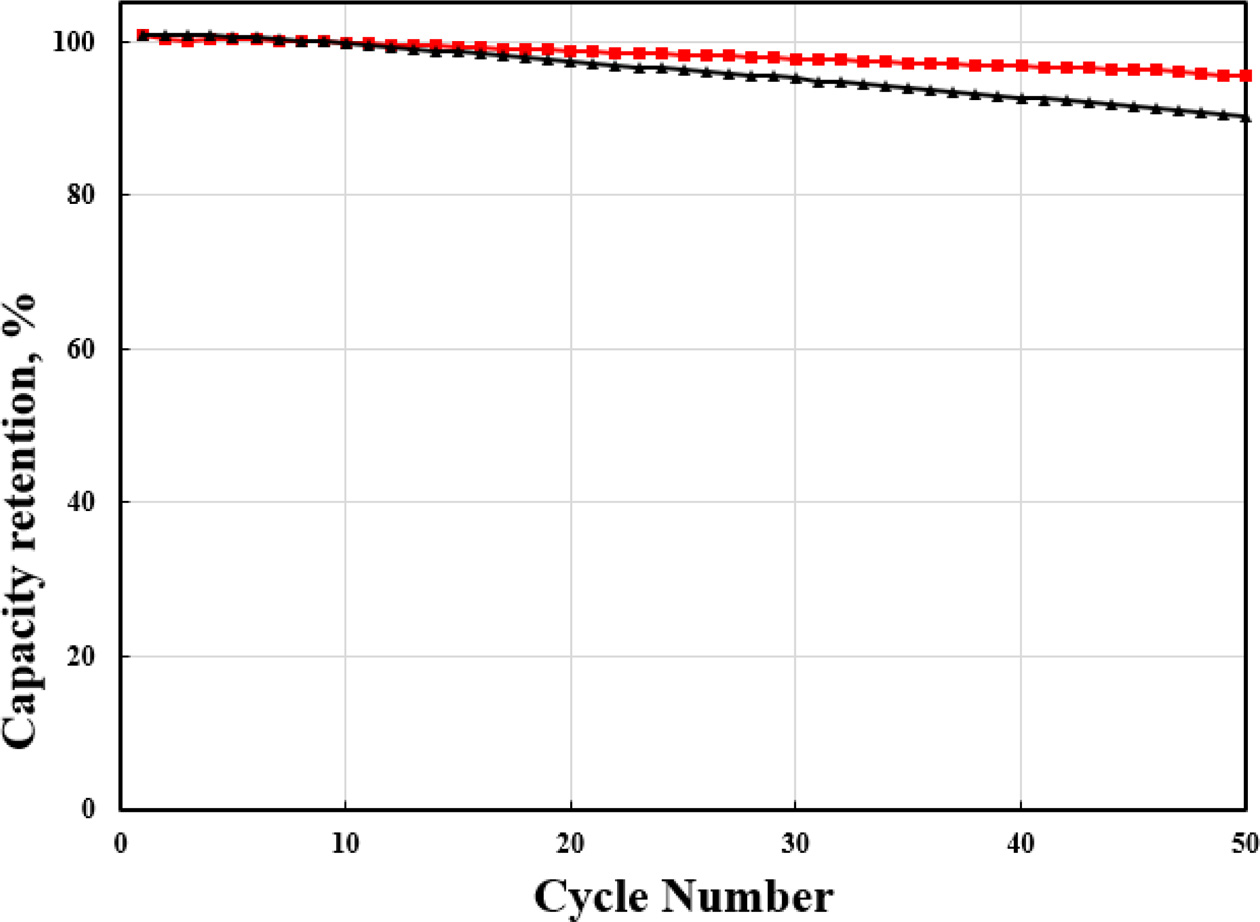
Fig. 6 shows the cyclability of pristine and Zr-doped
NCM91 at 0.5 C. The Zr-doped NCM91 show the superior cycle stability
compared to pristine NCM. Especially, 0.5 wt% Zr-doped NCM91
shows the highest capacity retention after cycle test.
The capacity retention of pristine NCM91 is 90.2% after 50
cycles. However, Zr-doped NCM display the 94.5% under the same condition. It is
closely related to the contribution of Zr doping,
resulting in reduction of charge transfer resistance of the
electrolyte/NCM91 interface and alleviation of transition metal dissolution in
NCM91 [4, 6, 9]. It can be inferred that Zr-doped NCM91 can maintain the
excellent structural stability or surface chemistry with the electrolyte under
long-term cycling. Yoon et al. reported that Li2ZrO3
layer can be formed on the surface of cathode. The protective Li2ZrO3
layer can be suppress the side reaction between electrolyte and NCM91
[12]. It is well known that the phase transition (H2→H3), which is one of the
main reason for the capacity fading, by lattice shrinkage along the c-axis,
leading to the volume change and micro-cracking [13]. Zr doping causes the
phase stabilization of NCM91, enables the particles to maintain the original
shape with accommodating the internal strain caused by the volume
change [12].
|
Fig. 1 XRD patterns of pristine and Zr doped NCM91. |
|
Fig. 2 FESEM images of the pristine and Zr-doped NCM91. |
|
Fig. 3 EDS mapping of Zr-doped NCM91. |
|
Fig. 4 Initial charge-discharge curves of pristine and Zr doped
NCM91. |
|
Fig. 5 Rate capability of pristine and Zr doped NCM91. |
|
Fig. 6 Cycle performance of pristine and Zr doped NCM91. |
In this paper, we successfully synthesized pristine and
Zr-doped NCM91 via solid-state reaction. Zr-doped NCM91 delivers superior
structural properties as well as electrochemical performances. The Zr-doped NCM91
with high crystallinity has smaller cation mixing compared to
that of pristine NCM91 and maintain its original shape. The electrochemical
performances of Zr-doped NCM91 outperforms pristine NCM91. This is because Zr
doping not only reduces cation mixing, but also creates a synergistic effect by
forming a Li2ZrO3 protective layer on the NCM91 surface.
Therefore, we can conclude that Zr doping into the NCM91 can be regarded as an
one of the effective way for high performance NCM91 cathode.
- 1. S.H. Lee, B.S. Jin, and H.S. Kim, Sci. Rep. 9 (2019) 17541.
- 2. J.W. Seok, J. Lee, T. Rodgers, D.H. Ko, and J.H. Shim, Trans. Electr. Electron. Mater. 20 (2019) 548–553.
-

- 3. S.H. Lee, H.S. Kim, and B.S. Jin, J. Alloy. Comp. 803 (2019) 1032-1036.
-

- 4. S.H. Lee, S. Lee, B.S. Jin, and H.S. Kim, Sci. Rep. 9 (2019) 8901.
-

- 5. J. Zhao, Z. Wang, J. Wang, H. Guo, X. Li, W. Gui, N. Chen, and D. Yan, Energy Technology 6 (2018) 2358-2366.
-

- 6. S.H. Lee, �G.J. Park, �S.J. Sim, �B.S. Jin, and H.S. Kim, �J. Alloy. Comp. 791 (2019) 193-199.
-

- 7. S.J. Sim, S.H. Lee, B.S. Jin, and H.S. Kim, Sci. Rep. 9 (2019) 8952.
-

- 8. S.H. Lee, S.J. Sim, B.S. Jin, and H.S. Kim, Mater. Lett. 270 (2020) 127615.
-

- 9. S.H. Lee, K.Y. Kim, and J.R. Yoon, NPG Asia Materials 12 (2020) 28.
-

- 10. Y. Lv, X. Cheng, W. Qiang, and B. Huang, J. Power Sources 450 (2020) 227718.
-

- 11. W. Yan, S. Yang, Y. Huang, Y. Yang, and G. Yuan, J. Alloy. Comp. 819 (2020) 153048.
-

- 12. S. Yoon, U.H. Kim, G.T. Park, S.J. Kim, K.H. Kim, J. Kim, and Y.K. Sun, ACS Energy Lett. 3 (2018) 1634-1639.
-

- 13. H.H. Sun and A. Manthiram, Chem. Mater. 29 (2017) 8486-8493.
-

 This Article
This Article
-
2020; 21(5): 592-595
Published on Oct 31, 2020
- 10.36410/jcpr.2020.21.5.592
- Received on May 14, 2020
- Revised on Jun 30, 2020
- Accepted on Jul 16, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Seung-Hwan Lee
-
Department of Advanced Materials Engineering, Daejeon University, Daejeon 34520, Republic of Korea
Tel : +82-42-280-2414 - E-mail: shlee@dju.kr







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.