- Chemical treatment to improve usable strength of Ion-exchanged cover glass
Hoikwan Lee*, Byeongbeom Kim, Yuri Kim, Minki Kim, Seungho Kim and Seongtaek Lee
Display Research Center, Samsung Display Co., Ltd., Giheung-gu, Yongin-city, Gyeonggi-do, Korea
Glass leaching, including
dissolution and hydrolysis, was introduced to mend surface flaws in
ion-exchanged glass. The leaching behavior on the glass surface was analyzed by
using a spectrophotometer, AFM, a confocal LCM, a surface stress meter and TEM.
The results revealed that a silica-rich nano layer was formed on the surface of
the glass after soaking in an acidic solution by dissolution of alkali ions
from glass surface, and the formed layer including the flaws on the surface of
the glass were completely removed after being exposed to an alkaline solution.
We also found that leaching an aqueous solution increased the mechanical
strength dramatically in ion-exchanged glass without degradation of surface
quality.
Keywords: Ion exchange, Cover glass, Usable strength, Chemical treatment, Silica-rich layer
Glass is now commonly used in many portable electronic
displays, mobile phones and tablets, as a protective cover for the devices. The
ion exchange process, Nullification of Surface Damage, is generally conducted
to improve the mechanical properties which are dependent on the compressive
stress at the surface and the depth of penetration of larger ions. Thus far,
the ion exchange method has been widely used for the strengthening of cover
glass because the generated compressive stress prevents crack propagation in
glass adequately. However, due to recent requirements for thin, light-weight
and unbreakable devices, the glass used is getting thinner and thinner,
and the ion exchange method has limitations to meeting the unbreakable
glass requirements due to unavoidable nano damages on the surface of the glass
which are more critical when the local bending is made by blunt impact [1-6].
In order to meet the requirements, functional coatings are
used on glass with organic and inorganic materials to minimize the stress
concentration in the crack tip. Teisseire et al. reported that
edge-strengthening of flat glass increased with acryl coating [7]. Also, there
are several reports with silica-like coating, diamond-like coating and
sapphire-like coating to increase the glass hardness as well as the glass
strength by reducing the role of stress corrosion at the crack tip that is
promoted by moisture at the surface [8-10]. However, the organic coating is
accompanied by hardness reduction as well as
reliability issues, and the inorganic coatings have some
side effects such as transmittance reduction, reduction of compressive stress
due to the relatively high processing temperature, cost increase, etc. The most
critical problem of the coating method is that it couldn’t be free from surface
damages due to the inability to mend surface damage thoroughly [11].
Chemical etching or healing, to remove surface damages and
bring back a pristine state, is studied; but it has not met the surface quality
required. Etching with hydrofluoric acid is a well-known and easy method to
increase glass strength by enlarging the radius of the crack tip [12-14].
However, the etched surface becomes very uneven and the crack maintains its
original depth as etching proceeds. The greatest disadvantage is that the
microcracks become visible etch pits. Thus it is not very attractive as a
commercial technology [15-17].
Therefore in this study, we introduced the chemical
cleaning method of using glass leaching to include glass
dissolution and hydrolysis. A silica-rich nanolayer is placed on
the surface of the glass reaching the lowest depths of the flaws, and it is
formed uniformly by exposing the glass to an aqueous acid solution and leaching
the alkali ions. The formed silica-rich nanolayer is then
removed completely by soaking the glass to an aqueous alkaline solution. The
formation of silica-rich nanolayer is confirmed and characterized by using UV-VIS
spectra, AFM, LCM and TEM. The thickness of nanolayer is varied with time,
temperature, and pH. The tested glass has shown excellent mechanical
performance without a trade-off among the strength, surface quality, and
hardness.
The samples of glass used in this study are commer- cially available alkali-aluminosilicate
(Corning Gorilla 3 & Gorilla 5 Glass, Corning, USA) specially designed for
ion exchange up to a great CS (Compressive Stress) and deep DOL (Depth of
Layer). The samples of glass were cut to desired size (typically mobile phone
with dimensions 70 mm × 150 mm) and put through a
mechanical machining and surface finishing process to make a 2D or 2.5D shape.
The samples of glass were strengthened by ion exchange
processes with the recipe provided by the glass maker. The Gorilla 3
sampleswere treated with a single step process and the Gorilla 5 samples were
strengthened by a 2-step process. Prepared samples satisfied all specifications
required for a cove glass.
Acidic solutions were prepared with the three different
acids (H2SO4 70% Hangseong Chem, CAS NO: 7664-93-9, HCl
35% Hangseong Chem, CAS NO: 7647-01-0, HNO3 60% SN Chem CAS NO:
7697-37-2) and distilled water. Specimens were immersed in the acidic solution
(60 liter) in a plastic bath. The solution was stirred with a
Teflon-coated stirrer and heated with a Teflon-coated heater during the
experiment, and the leaching behaviors were monitored as a function of the time
and temperature. The formed silica-rich nanolayer was removed with alkaline
solution prepared by using NaOH (Soda Flakes 98% YoungJin Chem.)
and distilled water.
The formation and removal of the silica-rich nanolayer
was confirmed and characterized by using spectropho- tometer (CM-3600d, Konica Minolta), AFM
(NX-20, Park Systems), LCM (Laser Con-focal Microscope), FSM (a surface stress
meter FSM-6000LE; Orihara, Toshima, Japan) and TEM. The thickness of the
silica-rich nanolayer was dependent on time, temperature, and
pH level of the solutions. Its effect on the mechanical property of
glass was tested with a dynamic impact test with a 150 g steel ball.
When ordinary glass was exposed to an aqueous solution,
alkali ions from the glass were extracted into the solution, and an alkali
deficient leached layer that is also a silica-rich nanolayer is formed on the
glass surface. The thickness of silica-rich nanolayer depends on the glass
composition and test conditions such as time, temperature and pH of the
solution [18-21]. One interesting finding is that the silica-rich nanolayer
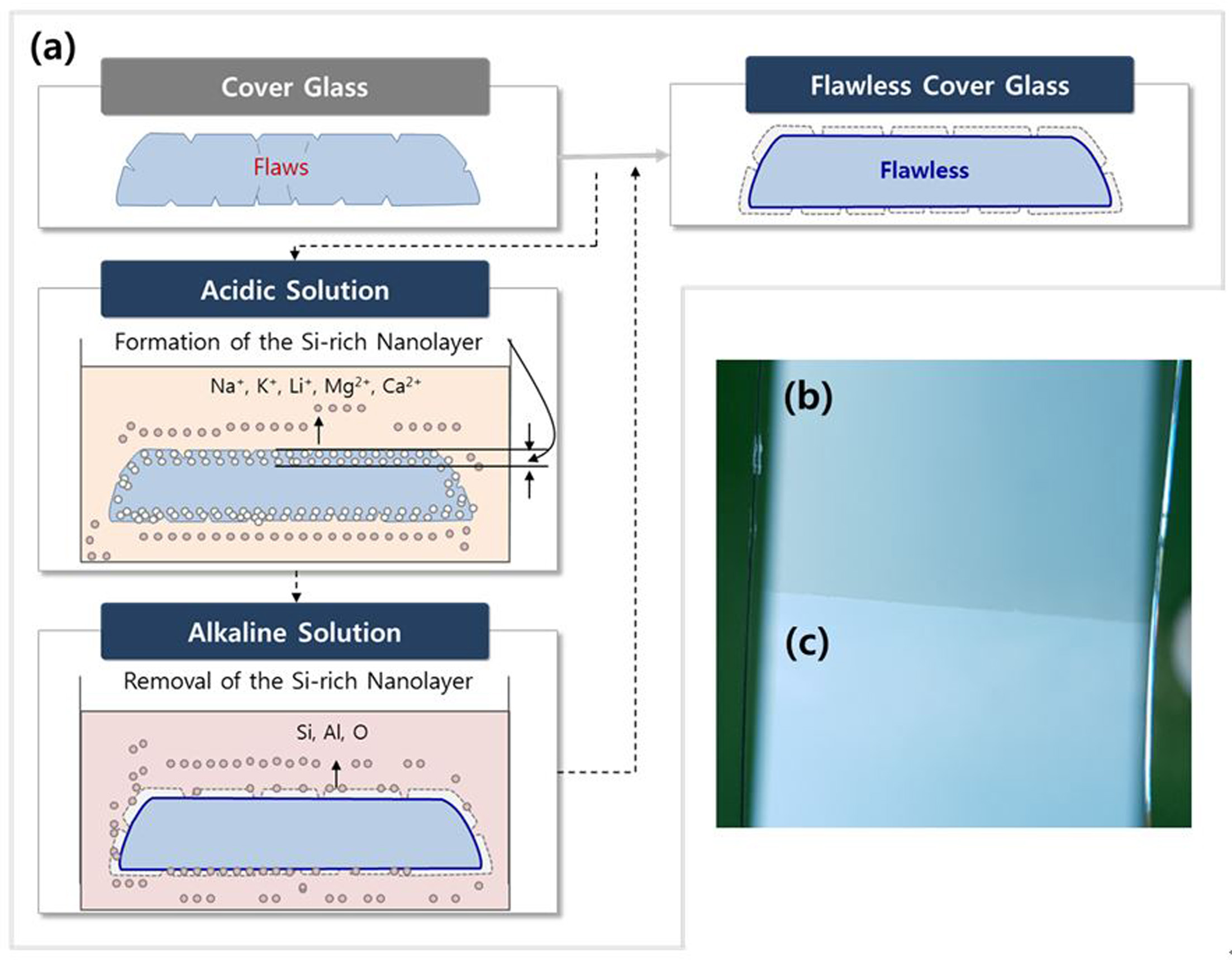
formed on glass surface became susceptible to decom- position in alkaline solution. Figure 1(a)
shows the processes when a sample of glass is exposed to an acidic solution as
well as alkaline solution. Here a silica-rich nanolayer was formed on glass
surface including the flawed areas with a thickness of 200~ 1000 nm in
order to improve the glass strength further. This thermodynamic prediction has
been verified by Shamy, Lewins and Douglas [22]. To observe the formation of
silica-rich nanolayer, half of the sample of glass was sealed with masking tape
after exposure to an acidic solution followed by an alkaline solution soak.
Figure1 (b, c) shows the top view of glass surface before and after alkaline
solution exposure. Fig. 1(b) indicates the silica-rich layer formed on the
glass surface after soaking it with an acidic solution, and Figure 1(c) shows
the silica-rich layer was removed with an alkaline solution.
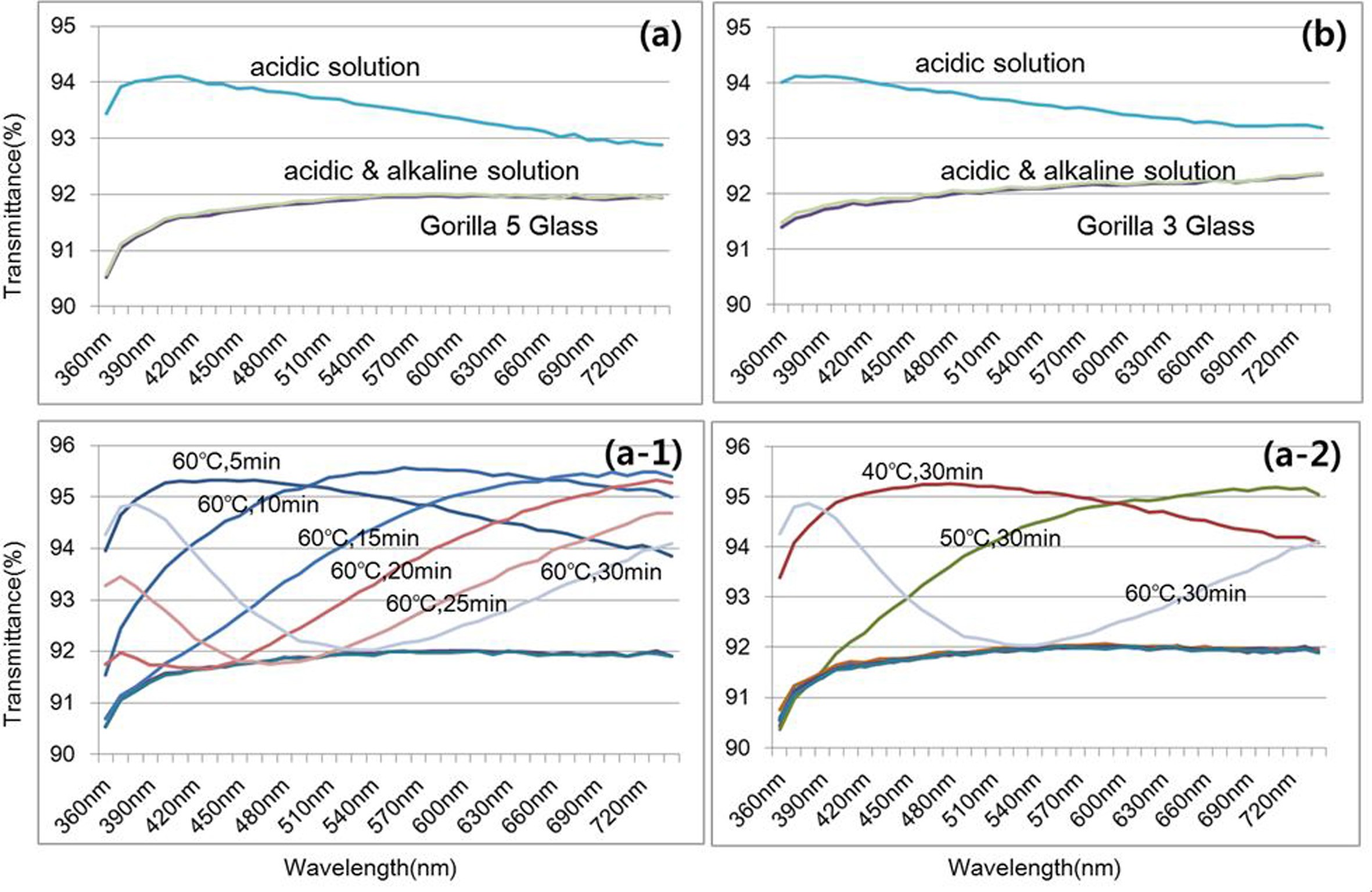
Transmittance spectra of the Gorilla 3 & 4 glass
samples are presented in Fig. 2(a, b) respectively. Both glass
samples show transmittance increase after exposure to an acidic
solution due to the formation of silica-rich nanolayer which is a relatively
low density that reduces the reflectance. The transmittance quickly recovered
to a pristine state by removing the silica-rich nanolayer in an alkaline
solution. Fig. 2(a-1, a-2) shows the time and
temperature dependence of the silica-rich nanolayer. The maximum
transmittance point moving to high wavelength means that
the silica-rich nanolayer is getting thicker with the increase of time. The
results indicate that temperature and time are important factors in deter- mining the thickness of the silica-rich
nanolayer, and the glass samples with relatively thick silica-rich
nanolayer easily return to a pristine surface after exposure to an
alkaline solution.
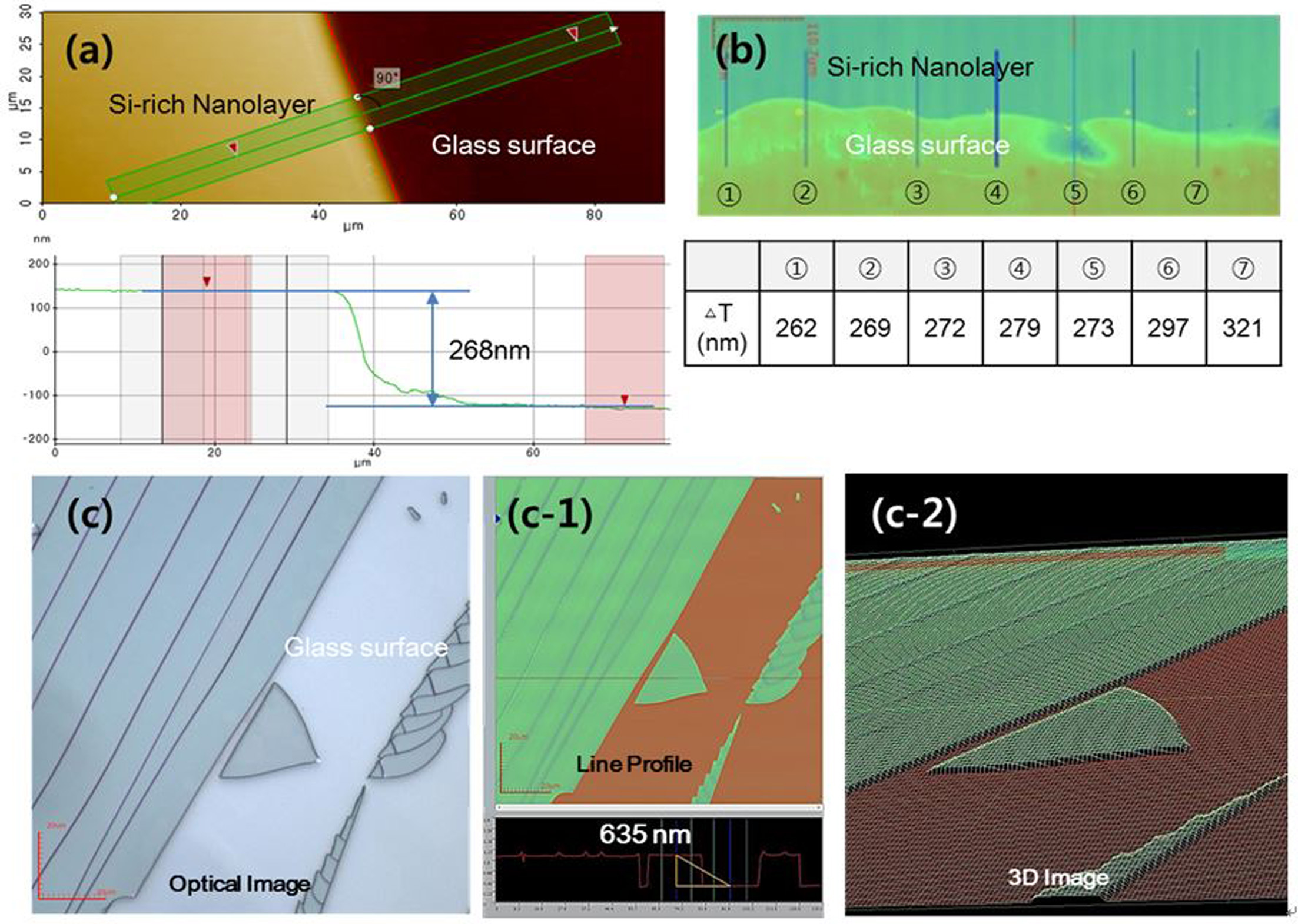
The measurements at the borderline were carried out with
the same samples in Fig. 1(b, c). As shown in Fig. 3(a), AFM indicates
268 nm as the thickness of the silica-rich nanolayer and this value was
confirmed with LCM measurement at 8 points. Mechanical impact with
a steel ball made the silica-rich nanolayer separate. As seen in Fig. 3(c, c-1,
c-2), approximately 635 nm thick silica-rich layer was separated
successfully, and the removed surface was as flat as pristine glass.
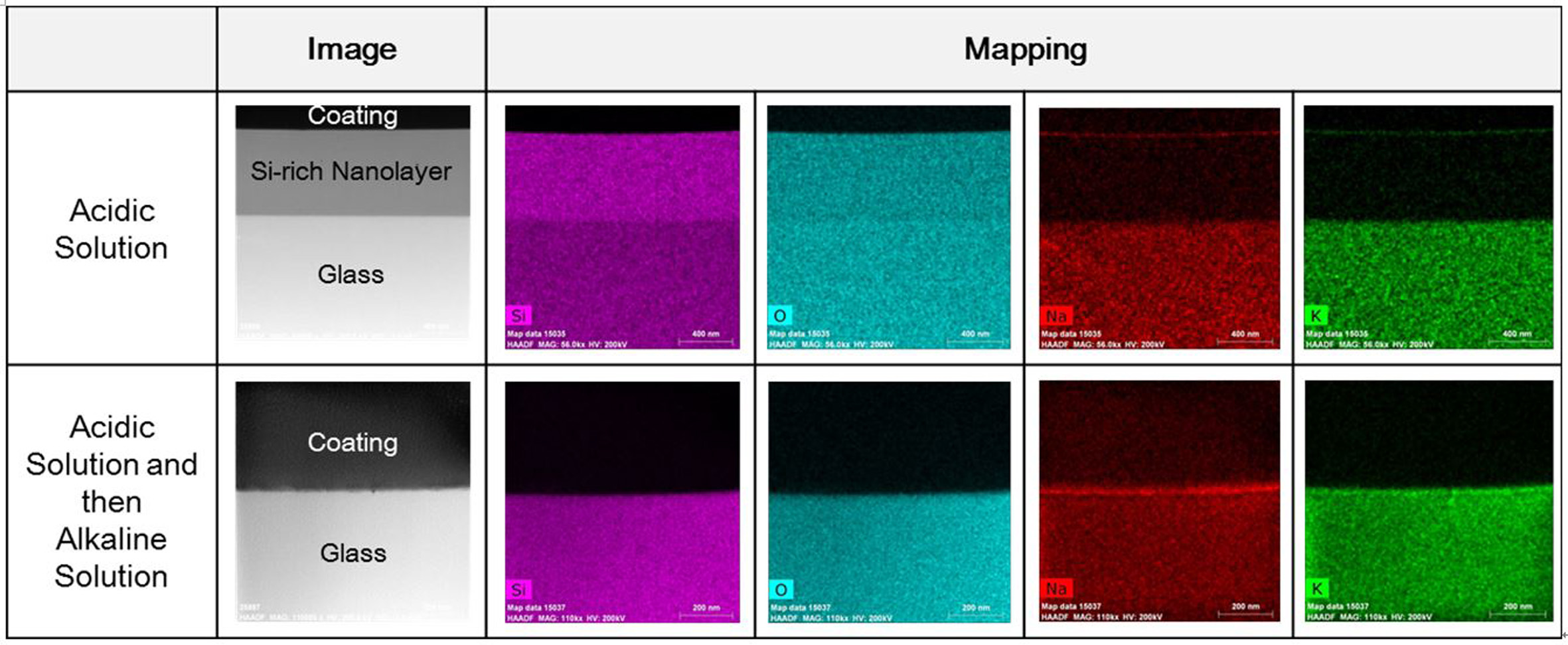
The glass compositions in the silica-rich layer were analyzed
with TEM EDS mapping and shown in Fig. 4. The results indicate that the
silica-rich layer consists of Si and O ions and Na and K ions were leached out
to a certain depth. It is confirmed again that the silicon-rich layer was
completely removed after exposure to an alkaline solution.
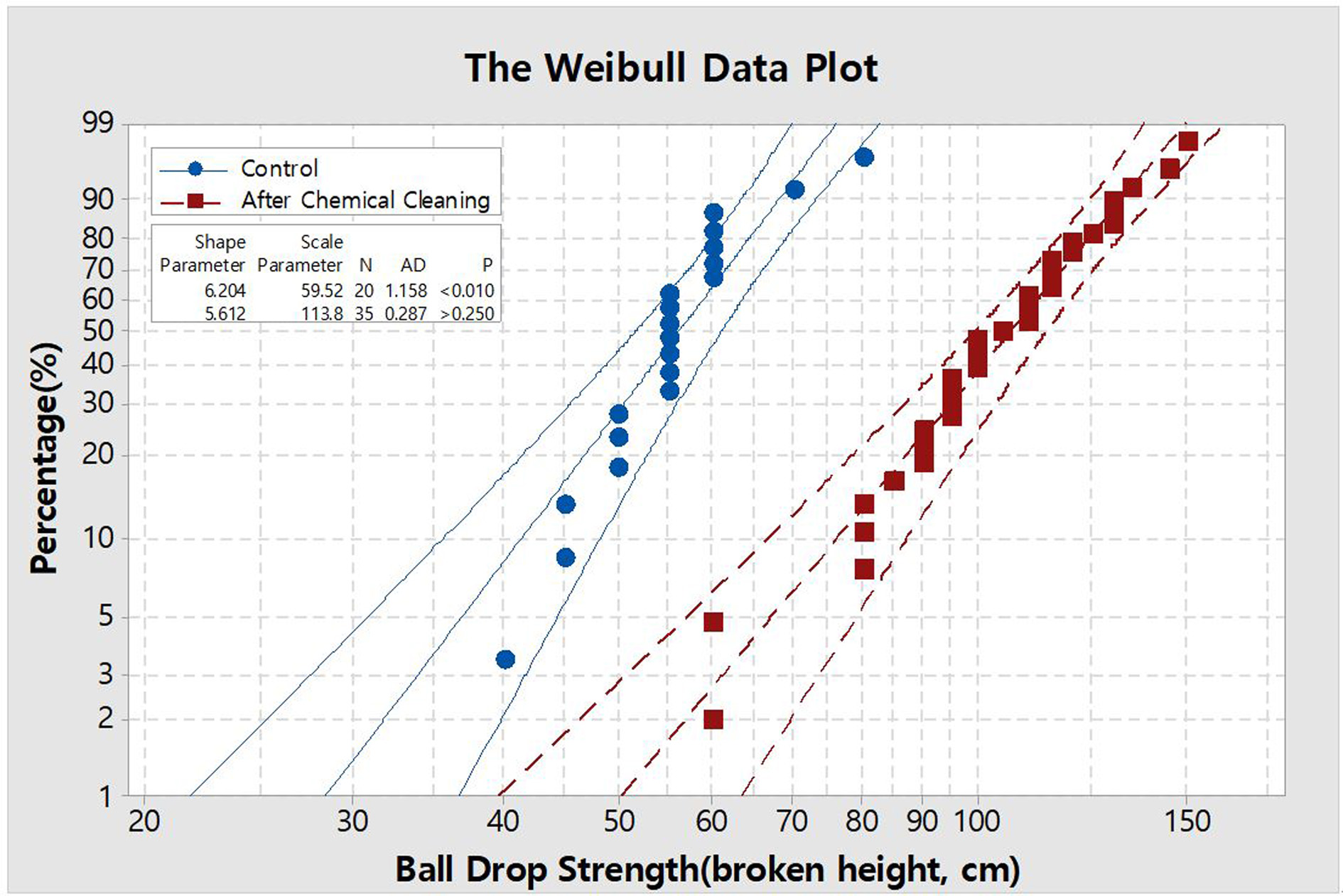
In order to prove the effect of the leaching process on
the mechanical strength of glass, the silica-rich nanolayer with a thickness of
300~400 nm was formed by exposing the sample to an acidic solution and
removed by dipping the sample in an alkaline solution. To measure the glass
surface strength before and after the leaching process, ball drop testing was
conducted by impacting the center of the glass surface with a 150 g steel
ball. Height was increased at intervals of 5 cm until the breaking point
was found. The results are presented with a Weibull plot as seen in Fig. 5. In
terms of the scale parameter of 63.2%, it is clear that the surface is
strengthened as the leaching process increases 1.9 times. The more interesting
point is that the surface quality was as good as pristine glass and there was
no known side effects caused by this process.
In this study, glass leaching was introduced to improve
mechanical property of chemically strengthened glass. A
silica-rich layer that reached flaw depths was formed on the surface of the
glass by exposing the glass to an acidic solution. The thickness of silica-rich
layer was controllable by adjusting the time and temperature of the solution.
The formation of a silica-rich layer was confirmed with naked eye, and the
thickness was measured by AFM and LCM. TEM mapping
revealed that the silica-rich layer was composed of Si &
O, and the layer was removed completely after being soaked in an alkaline
solution.
In order to see the effect of the leaching process on
glass surface strength, a ball drop test was performed with a 150 g steel
ball. The glass strength increased with the thickness of silica-rich layer and
saturated at 300 nm. When the silica-rich layer was thicker than
1000 um, the glass strength was decreased due to the loss of compressive
stress. The saturation point was selected in the range of 300~400 nm, and
the reduction of compressive stress was 30~40 MPa. An interesting point is
that this method doesn’t have trade off among the strength, surface quality,
hardness and long-term reliability. This work will serve as a foundation for
mending surface flaws in ion-exchanged glass.
|
Fig. 1 (a) Leaching process to form and remove the Si-rich Nanolayer (including flaws), (b) top view of glass surface (Si-rich Nanolayer)
after soaking in acidic solution, (c) top view of glass surface (Pristine surface) after soaking in acidic and alkaline solution. |
|
Fig. 2 Comparison of transmittance (a,b) of Gorilla 5 & 3 glass samples after exposing the glass samples to an aqueous acid solution and
alkaline solution respectively, transmittance variation as a function of soaking time (c) and temperature (d) in acidic solution. |
|
Fig. 3 Thickness of silica-rich nanolayer at the boundary (a) with AFM analysis and (b) with LCM analysis, (c) uniformity after removal of
silica-rich nanolayer. |
|
Fig. 4 TEM EDS mapping on fractured glass surface as a function of the chemical cleaning process. |
|
Fig. 5 Weibull plots for data sets of ball drop strength with 150 g steel ball; 95% confidence intervals. |
Leaching of glass made the silica-rich nano layer on
ion-exchanged glass, and removed it as well. The thickness of the silica-rich
layer was varied as a function of the glass soaking time and the temperature of
the acidic solution within the range of 100 nm ~1500 nm. The alkaline
solution caused the glass to have a pristine surface by removing the
silica-rich layer composed of Si and O. We also demonstrated that leaching can
improve the strength of ion-exchanged glass dramatically (up to two
times the original strength) without any degradation of surface
quality.
- 1. K. Kawamoto, T. Murata, S. Miwa, M. Ohji, and H. Yamazaki, SID DIGEST (2014) 1240-1243.
-

- 2. H. Ikeda, K. Kinoshita, M. Fukada, K. Kawamoto, T. Murata, K. Choju, M. Ohji, and H. Ymazaki, SID DIGEST (2014) 1365-1367.
-

- 3. D.R. Uhlmann and N.J. Kreidl, Glass Science and Technology: Academic Press (1980) 214-270.
-

- 4. A.K. Varshneya, INT. J. APPL. GLASS SCI. 1[2] (2010) 131-142.
-

- 5. F. Rehouma and K.E. Aiadi., Int. J. Commun. 4[1] (2008) 148-155.
- 6. A.K. Varshneya and W.C. LaCourse, Ceram. Trans. 29 (1993) 365-376.
- 7. J. Teisseire, D. Dalmas, S. Lohou, C.D. Silva, and E. Barthel, Int. J. of Fracture 170 (2011) 115-121.
-

- 8. C.R. Lin, D.H. Wei, C.K. Chang, and W.H. Liao, Phys. Procedia. 18 (2011) 46-50.
-

- 9. Rubicon Technology, Sapphir EX durable coatings. www.rubicontechnology.com
- 10. S.A. Mahadik, M.S. Kavale, S.K. Mukherjee, and A.V. Rao, Appl Surf. Sci. 2[257] (2010) 333-339.
-

- 11. D. Hanft, J. Exner, M. Schubert, T. Stocker, P. Fuierer, and R. Moos, J. Ceram, Sci. Tech. 06[03] (2015) 147-18
- 12. T. Suratwala, W. Steele, L. Wong, M.D. Feit, P.E. miller, R. Dylla-Spears, N. Shen, and R .Desjardin, J. Am. Ceram. Soc. 98[8] (2015) 2395-2402.
-

- 13. L.M. Cook, J. Non-Cryst. Solids 120[1-3] (1990) 152-171.
-

- 14. T.P. Dabbs and B.R. Lawn, J. Am. Ceram. Soc. 65[3] (1982) C37-C38.
-

- 15. E.K. Pavelchek and R.H. Doremus, J. Maer. Sci. 9 (1974) 1803-1808.
-

- 16. B.A. Proctor, Appl. Mater. Res. 3 (1964) 28-37.
- 17. V.A. Ryabov and V.V. Kupfer, Glass Ind. 51 (1970) 268-271.
- 18. L.L. Hench, J. de Phys. C9 (1982) 625-636.
-

- 19. M.M. Smedskjaer, J. Deubener, and Y. Yue, Chem. Mater. 21 (2019) 1242-1247.
-

- 20. M.D .Bardi, R. Wiesinger, and M. Schreiner, J. Non-Cryst. Solids. 360 (2013) 57-63.
-

- 21. F. Farges, M.P. Etcheverry, A. haddi, P. trocellier, E. Curti, and G.E. Brown, AIP Conference Proceedings 882 (2007) 44-51.
-

- 22. T.M. El-Shamy, J. Lewins, and R.W. Douglas, Glass Technology 3[13] (1972) 81-87.
 This Article
This Article
-
2020; 21(5): 547-551
Published on Oct 31, 2020
- 10.36410/jcpr.2020.21.5.547
- Received on Mar 16, 2020
- Revised on Apr 20, 2020
- Accepted on May 4, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Hoikwan Lee
-
Display Research Center, Samsung Display Co., Ltd., Giheung-gu, Yongin-city, Gyeonggi-do, Korea
Tel : +82-031- 5181-6566
Fax: +82-031-8000-6822 - E-mail: hoikwanlee@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.