- Utilization of sugar processing filter cake instead of calcite in production of anorthite based ceramics
Vacide Selin Kayaa,b and Mucahit Sutcuc,*
aIzmir Katip Celebi University, The Graduate School of Natural and Applied Sciences, Izmir 35620, Turkey
bEge University, Aliağa Vocational Training School, Metallurgy Program, Izmir, Turkey
cIzmir Katip Çelebi University, Faculty of Engineering and Architecture, Department of Materials Science and Engineering, İzmir 35620, Turkey
In recent years, the reuse of
industrially generated residues in ceramic bodies has been an important issue.
Reuse of industrial waste products is not only environmental responsibility,
but also cost reduction and efficient use of raw materials. The aim of the
study was to investigate the possibility of using press filter cake (PFC)
instead of calcite in the production of anorthite-based ceramics. The PFC is an
industrial solid waste, which is a by-product in the production of sugar from
sugar beet. This solid waste quantity is about one million ton per year in
Turkey. PFC was evaluated as alternative raw material in the preparation of
anorthite based ceramic bodies. In this study, anorthite based ceramics were
produced by using two different types of calcium oxide source. While PFC and
calcite were used as CaO source, clay was used as the source of aluminum
silicate. Compositions were prepared from 30% calcium oxide sources and 70% clay
by weight. The ceramic bodies were sintered at different temperatures from
1000 oC to 1200 oC. The micro-porous structure
of ceramics were obtained by burning of organic substances and by decomposing
of carbonate compounds of the PFC wastes during firing process. The differences
between the physical, mechanical and microstructural properties of anorthite
based ceramics produced from two different CaO sources were investigated. Also,
their phase analysis results were compared. While the specimens with 30 wt%
PFC addition fired at 1200 oC contained anorthite (CaO·Al2O3·2SiO2)
and cristobalite (SiO2) as major phase, the specimens with 30wt%
calcite addition fired at same temperature included anorthite as major phase
and minor secondary phases such as cristobalite and quartz.
Keywords: Sugar processing wastes, Firing, CAS ceramics, Phase analysis, Microstructure
Anorthite (CaO·Al2O3·2SiO2)
is one of the crystalline phases of the ceramic material,
which has a calcium-aluminum-silicon oxide (CAS) composition. Due to its low
thermal conductivity, low theoretical density, high melting point, low
dielectric constant, this ceramic composition has a great
potential for using in insulation, refractory and electronic
applications. [1-6]. Anorthite-based ceramics can be commonly obtained as
glass-ceramics or ceramics, sometimes by controlled
crystalli- zation of
glass, or sometimes from kaolin and calcium carbonate mixtures. These ceramics
produced in dense and porous forms are preferred in many different applications
due to their interesting properties. For instance, the
mechanical strength of clay-based ceramics can
be increased by forming anorthite crystals embedded in the
matrix in their microstructures when sintered even at low
temperatures using clay and calcite mixtures. In general,
in the formation of such a microstructure during thermal transformations, the
reaction sequence takes place as metakaolinite-gehlenite-anorthite [7]. Many
researchers have used low-cost natural raw materials such as clay, kaolin,
calcite, dolomite, quartz and feldspar to prepare these ceramics. In many
studies, the synthesis of anorthite powders and the production of dense
anorthite ceramics have been extensively studied using different methods such
as sintering of raw materials, mechano-chemical processes
and the use of different sintering aids [8-16]. Kurama et al. [8] evaluated the
effect of different calcium oxide sources in order to produce anorthite
ceramics. Also, sintering behaviors of anorthite based porcelain bodies was
investigated by using additives such as wollastonite and dolomite
as a calcium source [17, 18]. Moreover, the formation of anorthite crystalline
phase was deter- mined in
glass-ceramic glazes derived from industrial wastes such
as blast furnace slag and fly ash [19]. In addition to research on the
development of dense anorthite ceramics, some studies have recently attracted attention
on the development of porous anorthite ceramics from
different sources of raw materials, especially industrial
wastes. Porous anorthite ceramics are generally utilized as
lightweight thermal insulation materials in refractory applications [12, 13].
Some researchers have reported their findings in their effort to develop the
properties of anorthite based lightweight insulating materials produced by
using of some wastes such as eggshell, marble powder, recycled paper processing
residues as a source of calcium oxide [11-14]. The use of industrial wastes as
a source of calcium for the anorthite composition as well as the pore former in
the production of porous anorthite ceramics has gained importance due to its
economic, environmental and energy saving advantages [8, 11, 14, 20-24].
In this study, press filter cake (PFC), an industrial
solid waste of sugar factory, was used to produce anorthite based ceramics. The
PFC is an industrial waste that is exposed in the carbonation treatments in
sugar factories. It contains organic and inorganic substances and consists
mainly of calcium carbonate (CaCO3). This residue could not be reuse
for the sugar process. The resulting wastes are either drained into the
environment or stored at the factory site. This process negatively affects the
quality of the rivers in terms of environment and can damage the soil in the
region due to the high calcium carbonate content. PFCs stored at the factory
site also cause various problems in terms of waste management. As a result of
industrial processes performed in the sugar factory in Turkey it is produced
about one million tons of PFC wastes annually [25]. Therefore,
the different recovery or reassessment studies are
performed to overcome this problem. This waste from the sugar beet industry has
been used by some researchers in the production of anorthite-based
ceramics as a source of calcium oxide [21-24]. Using this waste
together with a local clay, El-Maghraby et al. [21] obtained samples with a
dominant anorthite crystal phase, bending strength of about 25 MPa, and a
relative density of 64.5%, after sintering at high temperatures above
1200 oC. Naga et al. [22] investigated the effects of the ratio
of crystalline phases on the physical, mechanical,
electrical and thermal expansion coefficient properties
of the cordierite/anorthite ceramic composites prepared by
using sugar beet filter cake and talc. In another
study, Naga et al. [23] prepared anorthite powder by using
sugar beet industry waste and kaolin, then used it to prepare anorthite-alumina
composites. The physical and mechanical properties of these composites were
studied as a function of the amount of added alumina. Moreover, Man et al. [24]
prepared the porous bricks that consist of 80 wt% diatomite, 20 wt% sugar
filter cake, which have open porosity of 50% and sufficient flexural strength
of 10 MPa after firing.
The aim of the study was to investigate the possibility
of using PFC as a source of calcium and pore former to replace
calcite in the manufacture of a porous anorthite-based
ceramic product. In this study, the physical, mechanical and also,
microstructural characteristics of the produced samples were investigated.
The sugar filter cake is obtained from the Eskişehir Sugar
Factory (in Turkey), which produced 50,000 tons of sugar cake per year.
Clay was provided by Omya Inc. (Kırşehir, Turkey) and calcite was supplied by
Kale Mine Company (Çanakkale, Turkey). The raw materials were analyzed for
particle size, chemical composition, phase content and
thermal behavior. Particle size distribution of the raw materials
was measured by Malvern Mastersizer 3000. An Energy Dispersive X-ray
Fluorescence Spectrometer (XRF, Rigaku NEX CG Model) was used for determining
their chemical com- position. The phase
compositions of the raw materials were identified by X-ray diffraction (XRD,
Bruker D2 Phaser) with Cu tube of 1,54 Å at voltage of 30kV. Thermogravimetric
analysis (TGA) of the raw materials was carried out under
nitrogen atmosphere at heating rate of 10 oC/min using the TA
Instruments TGA-SDT Q600 equipment. The particle morphology of raw materials
was observed by a scanning electron microscope (SEM, Carl
Zeiss 300VP). Before SEM observation, all samples were gold coated.
The mixture compositions composed of 30% PFC + 70%
clay (CP mixture coded), and 30% calcite + 70% clay (CC mixture
coded) by weight. The slurry of batch compositions were prepared at
1215 g/l density and mixed with rotational speed of 200 rpm for
45 min to provide homogeneous blend. Then, the slurries were dried at 100 oC
for 24 h and ground in a mortar to obtain powder mixtures. The mixtures
were uniaxially compacted in form of cylindrical pellet with 20 mm
diameter at hydraulic press under a pressure of 25 MPa. After the samples were
dried at 100 oC for 24 h, they were fired at 1000, 1050,
1100, 1150 and 1200 oC for 2 hours to obtain anorthite (CaO·Al2O3·2SiO2)
as major crystalline phase.
The fired samples were characterized for their physical
properties, crystalline phases and microstructural pro-perties as well as compressive strengths and
thermal conductivities. The physical properties of fired samples such as bulk
density, apparent porosity and water absorption were evaluated by the water
immersion method according to ASTM C20 [26]. Crystal phase formations and
microstructures in the fired samples were examined with XRD and SEM,
respectively. Compressive strength of the samples was measured by Shimadzu
testing machine with 100 kN capacity at a crosshead speed of
0.5 mm/min. For measuring the thermal conductivity of samples, C-Therm
Thermal Conductivity Analyzer was utilized.
Characterization
of the raw materials
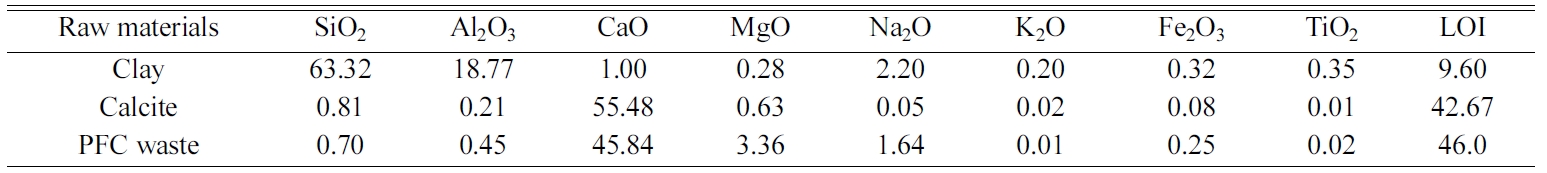
Table 1 shows the chemical composition of raw materials.
The loss on ignition values of clay, calcite and PFC waste were measured as
9.60, 42.67 and 46.0% by weight, respectively. The clay raw material mainly
includes about 63.3% SiO2 and 18.8% Al2O3. It
contains also minor constituents such as 2.2% Na2O and 1% CaO.
Purity of the commercial calcite is quite high. The content of PFC waste has
mainly calcium oxide content as well as minor amounts of the oxides of
magnesium and sodium.
XRD analysis was performed to identify the mineral phases
found in raw materials. Because these phase definitions are important to
identify certain phases that are likely to occur after firing the
ceramic compositions. Fig. 1 shows the XRD patterns of clay, calcite and
PFC waste. The analysis result of clay raw material indicates that quartz (SiO2)
was the major crystalline phase as well as kaolinite (Al2O3·2SiO2·2H2O).
The main crystalline phase of calcite raw material is calcium carbonate (CaCO3),
while PFC waste was observed as magnesium-calcium carbonate phase.
Fig. 2 indicates the TGA results of the raw materials.
According to the TGA curves, total weight loss of clay raw material is 10.42%
after firing at 1000 oC, the significant mass loss is due to
the dehydroxylation of kaolinite between 400 oC and 600 oC.
For the calcite raw material, TGA analysis showed that there is only an
endothermic reaction (total weight loss of 43.45%) in the range of 600 to
800 oC, which may be due to the thermal decomposition of CaCO3
into calcium oxide and carbon dioxide. In PFC waste, the total mass loss is
48.4%. The carbonate decomposition that occurs between 600 oC
and 800 oC appears to be less (about 38.4%) compared to calcite
raw material. In addition, it is observed that the removal of physical water
and organic compounds that are likely to be present in the waste before the
decomposition of carbonates is carried out at 100 oC and
300-500 oC, respectively.
Fig. 3 indicates the log-normal particle size
distributions of the raw materials and the curve of cumulative distribution is
also presented. The average particle sizes of clay, calcite and PFC appear to
be about 5 µm, 10 µm and 15 µm, respectively. The clay particle
sizes are less than 50 µm, while the calcite is less than 100 µm. PFC
has a bi-modal particle size distribution curve; in addition to particles
smaller than 60 µm, a small amount of agglomerated or coarse particles
(<600 µm) are also present. It is known that particle size fineness is
an important parameter for the rapid reaction of powder mixtures
during firing process.
Fig. 4 shows the particle morphology of raw materials. It
has been observed that the raw materials have some agglomerated powders
composed of fine primary particles less than 5 µm. The SEM images confirm
the results of particle size analysis. The particle morphology of PFC wastes
appears to differ from the calcite raw material. It is also clear that calcite
particles have sharper edges than that of PFC waste.
Characterization
of the fired samples
The physical, mechanical and thermal properties of the
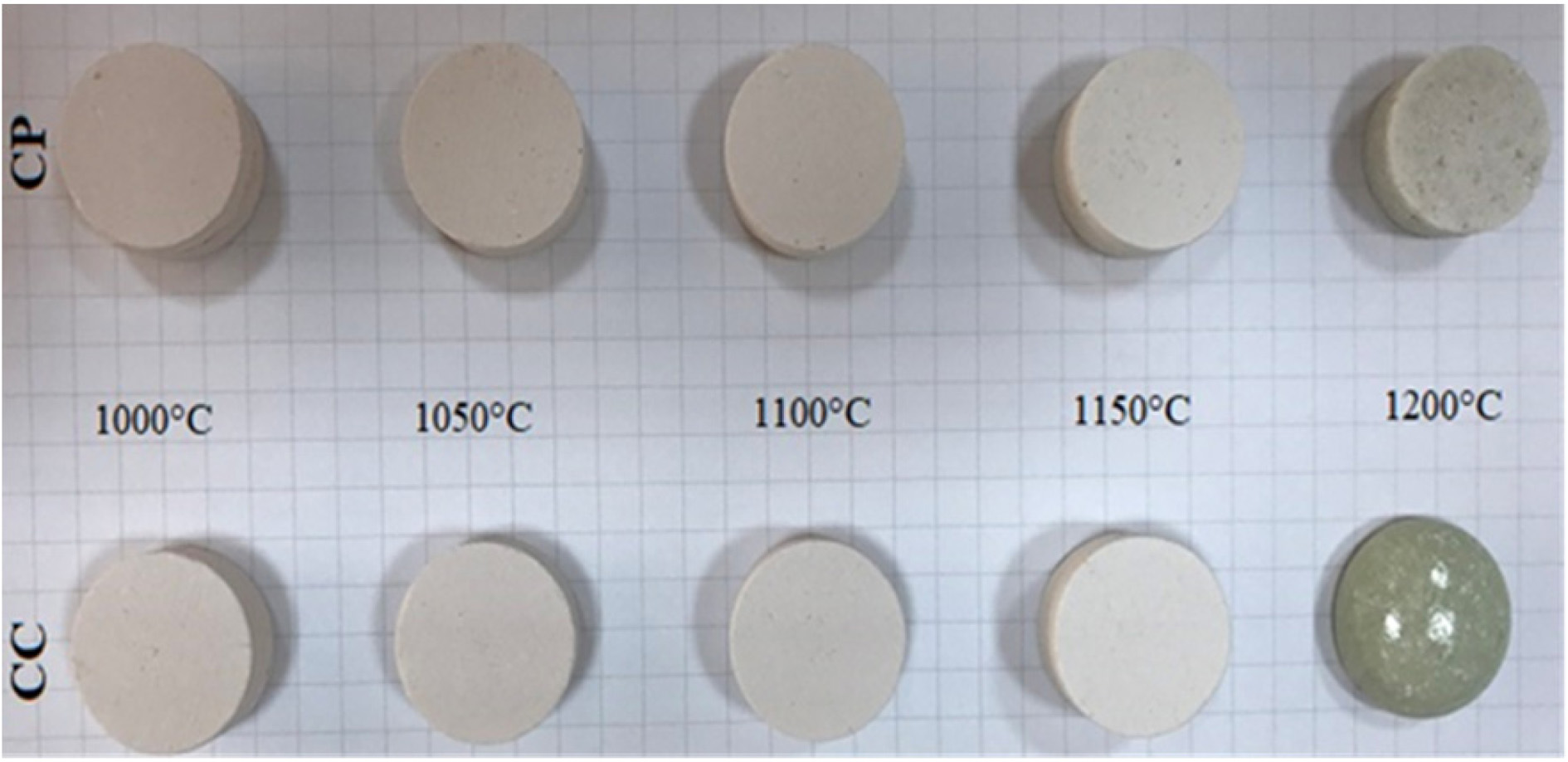
fired samples were characterized. Fig. 5 shows the photograph of pellet samples
fired at different temperatures. From the image of the fired samples, it can be
seen that the firing behavior of the two different calcium carbonates additive
admixtures is different at the applied temperatures.
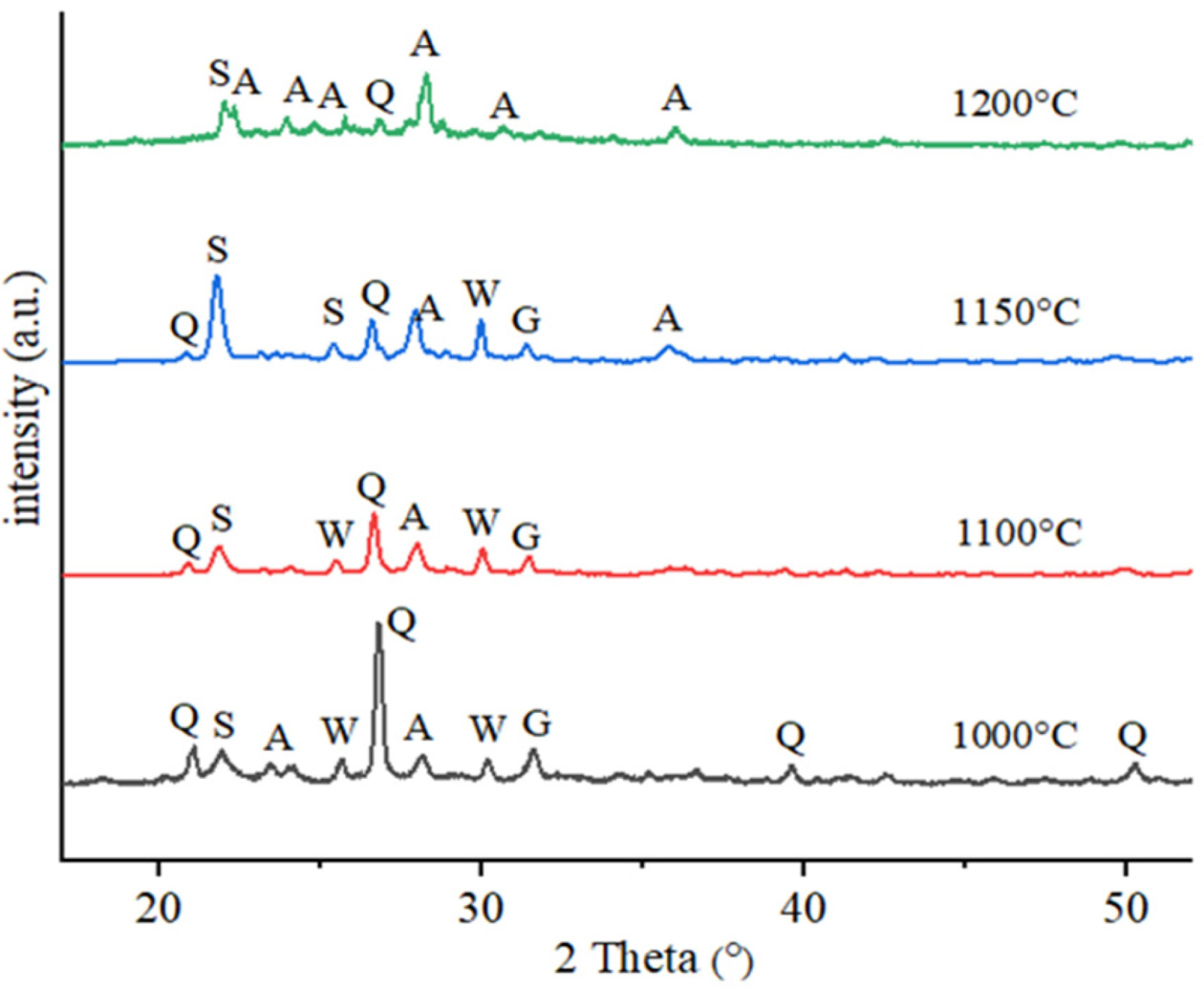
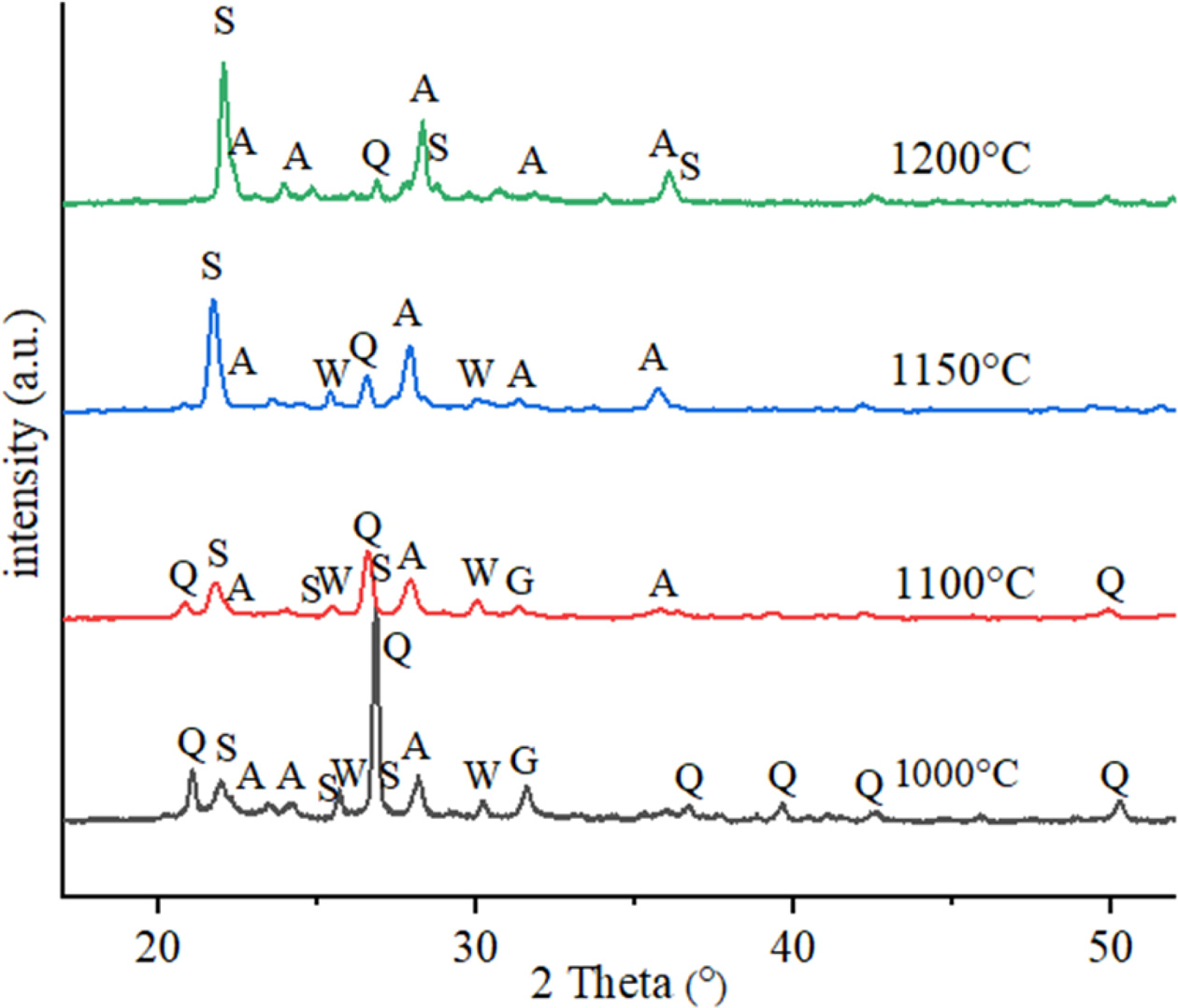
Figs. 6 and 7 present XRD spectra acquired from CC (30%
calcite + 70% clay) and CP (30% PFC + 70% clay) samples fired at
1000-1200 oC, respectively. The XRD patterns from CC and CP
samples show the characteristic peaks of quartz as major phase at low firing
temperature of 1000 oC. Also, in the both com- position fired at 1000 oC,
it was observed that the calcium alumina silicate (CAS) crystalline phases such
as anorthite and gehlenite started to form. Gehlenite and wollastonite peaks
appeared in the samples fired at 1000 and 1100 oC, which
completely transformed to anorthite at 1200 oC. At high firing
temperatures, especially at 1200 oC, it was observed that peaks
of quartz have decreased while characteristic peaks of anorthite and
cristobalite have increased. At 1200 oC, in the CC composition
samples, the major crystalline phase is anorthite as well as glassy phase. Due
to the low percentage of CaO in the CP composition, the main crystalline phase
is cristobalite, and followed by anorthite crystals. Gehlenite and wollastonite
phase peaks were dissappared at 1200 oC. The formation of the
anorthite phase in CP sample is more pronounced than CC sample at 1150 oC.
While the CC sample began to partially melt at 1200 oC, the
anorthite crystals became evident with the glassy phase and the silica in the
melt partially dissolved and decreased.
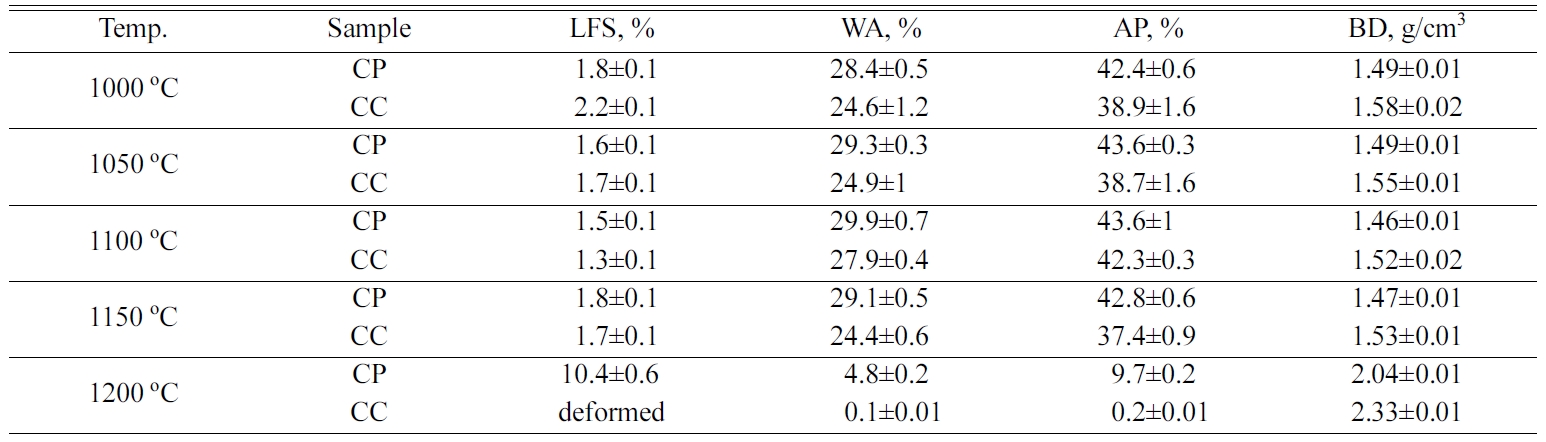
Physical properties of samples such as linear firing
shrinkage, apparent porosity, bulk density were deter- mined from average of three samples for each
com- position and firing temperature.
Table 2 gives linear shrinkage of the samples versus temperature. It was observed
that the linear firing shrinkage (LFS) values of the samples partially decreased
from 1000 to 1100 oC. The reason for this decrease may be the
result of the quartz crystals dissolving and thus the increasing of the CAS
crystal phases. The CC and CP samples tend to have larger shrinkage value from
1150 to 1200 oC, and also show a considerable liquid phase at
1200 oC.
While the apparent porosity ratios decreased, the bulk
densities of the fired samples increased with rising to 1200 oC.
Because of partial vitrification and pore closure, the CC and CP samples
indicated a rising in density values at 1200 oC. It was
observed that the CP samples demonstrated more stable behavior according to the
CC samples. The apparent porosity values of CP samples are partially higher
than that of CC samples. The CC samples, which contain anorthite as major
phase, vitrified at 1200 oC and their apparent porosity values
reduced to almost zero.
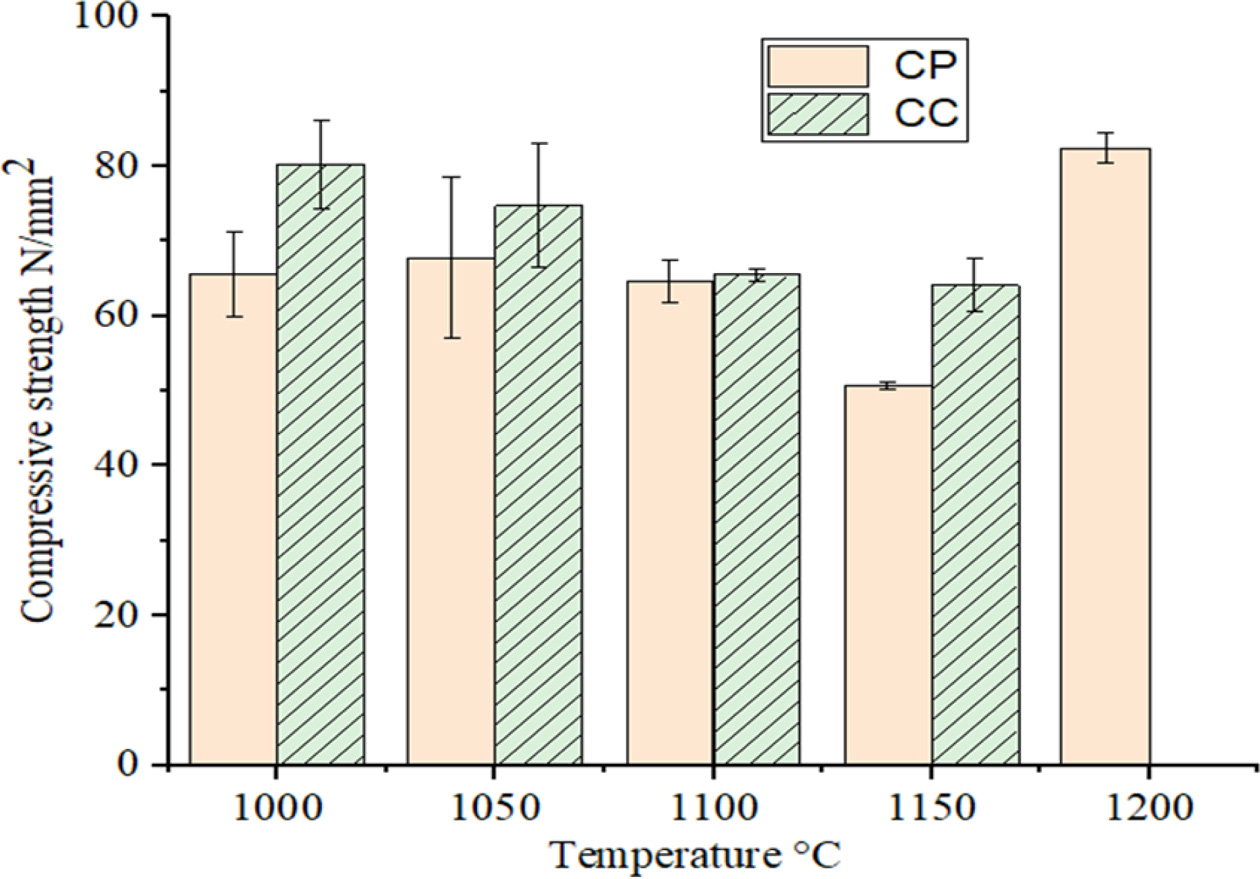
The compressive strength results of the CP and CC samples
which were fired from 1000 to 1150 oC are shown in Fig. 8. The
results are ranged from 63 to 50.7 MPa and 80.3 to 64 MPa,
respectively. By increasing of firing
temperature up to 1150 oC of the both samples,
the compressive strength values partially decreased. In the samples fired at
1200 oC, the strength of CP sample showed an increase trend to
about 82 MPa, however, the strength of CC sample was not measured because of
melting of the samples.
Thermal conductivity values for each composition and
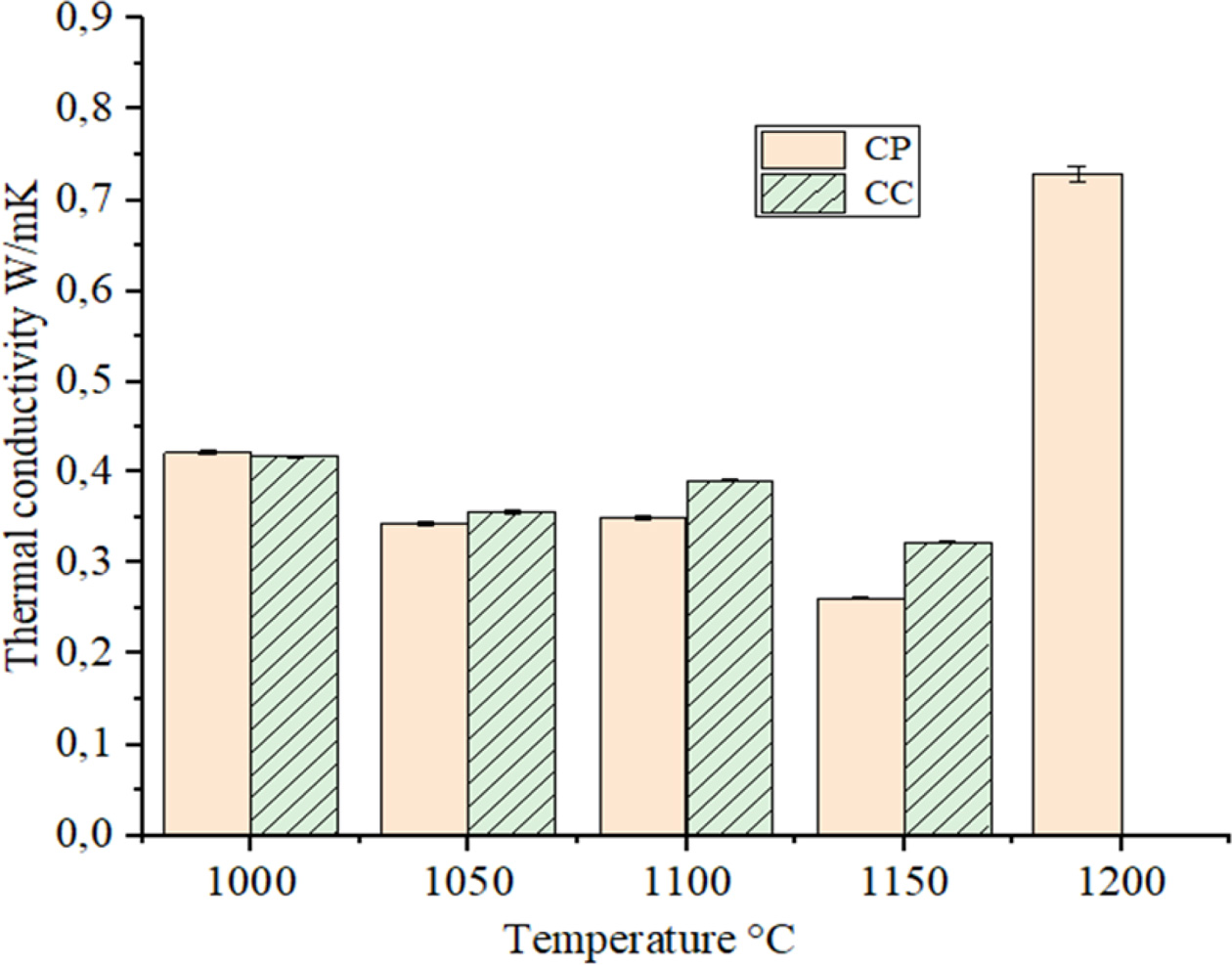
firing temperature were gotten from ten measure- ments of one sample. As seen from Fig. 9,
the thermal conductivity values of the CP and CC samples ranged from 0.42 to
0.8 W/mK. By increasing the firing temperature up to 1150 oC
of the both samples, the thermal conductivity values showed a decreasing trend.
Thermal conductivities of the CP samples decreased from 0.42 to 0.25 W/mK.
These results are promising for use as thermal insulation bricks of the ceramic
samples produced from sugar processing filter cake waste. In the samples fired
at 1200 oC, the thermal conductivity values of CP sample showed
a rapid increase to about 0.73 W/mK. This case is a result of partial
vitrification and densification. The thermal conductivity values of CC sample
was not measured because of melting the samples at 1200 oC.
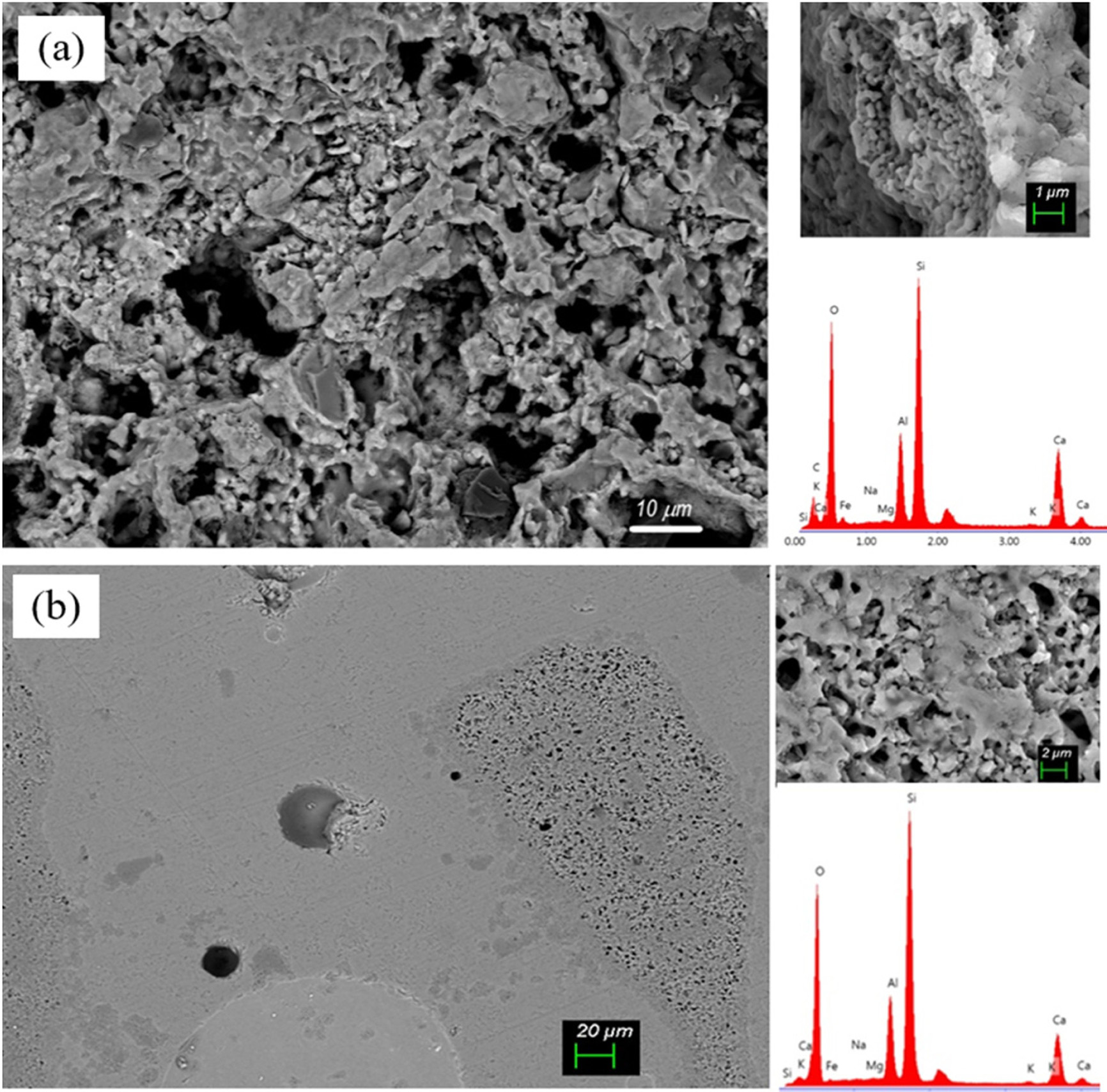
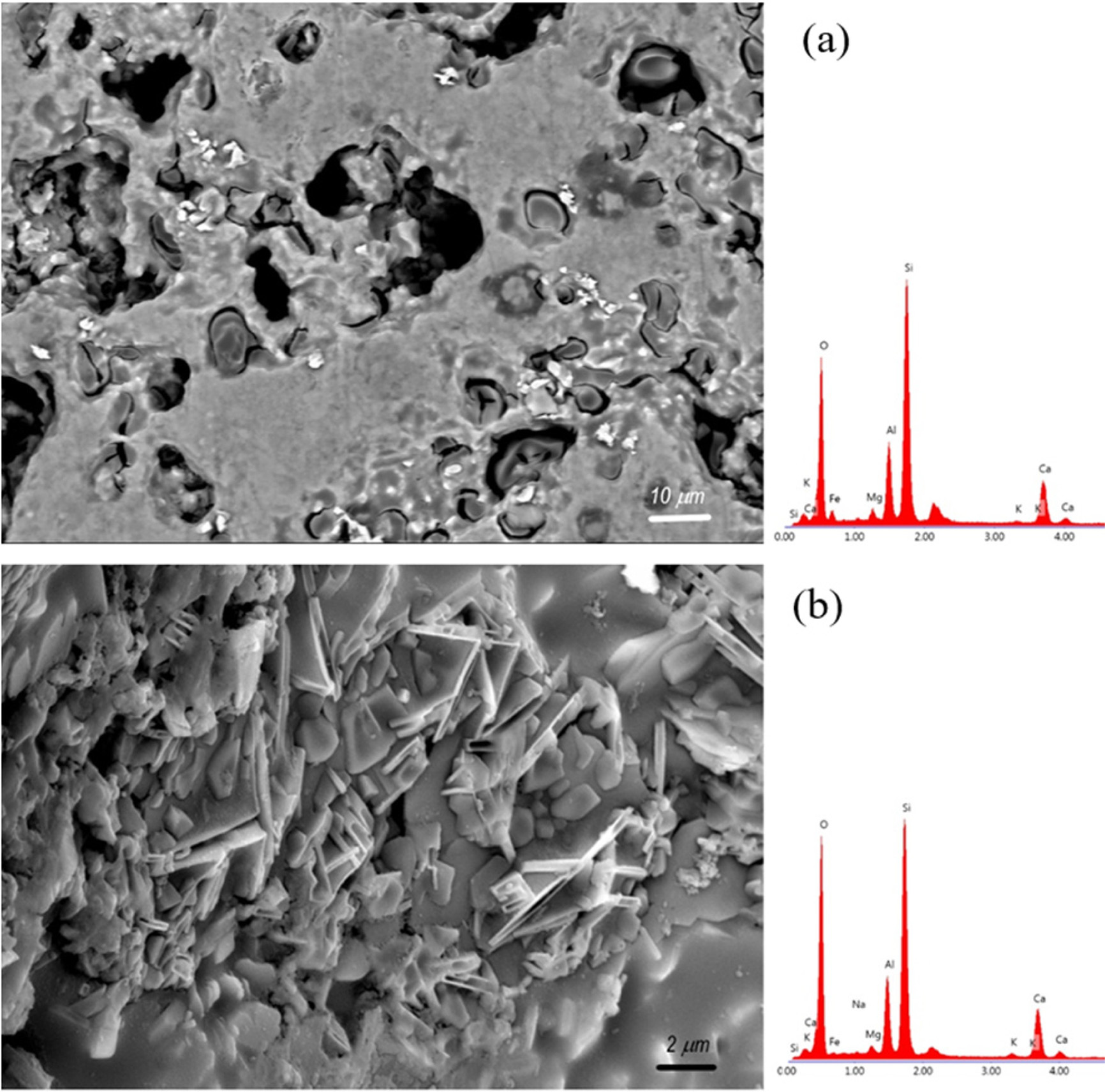
Figs. 10 and 11 show SEM micrographs of the etched
surfaces of the ceramic samples. In the figures, it was observed that the CC
and CP samples fired at 1150 oC have a porous structure. Also,
cristobalite and quartz particles in the ceramic matrix are present as
spheroidal crystals. The ceramic matrix is formed from anorthite crystals which
appear as plate-like and sub-micronfine particles. In the CP sample fired at 1150 oC,
it is observed that the silica particles embedded in the anorthite phase have
voids around the particles due to the thermal expansion difference from the
ceramic matrix. In the CC sample fired at 1200 oC, a dense
structure as well as local porous regions are observed in the structure. In the
CP sample fired at 1200 oC, plate-like
anorthite crystals are observed in the structure. According
to EDS results taken from the SEM images, the ceramic bodies have similar
Ca-Al-Si-O content.
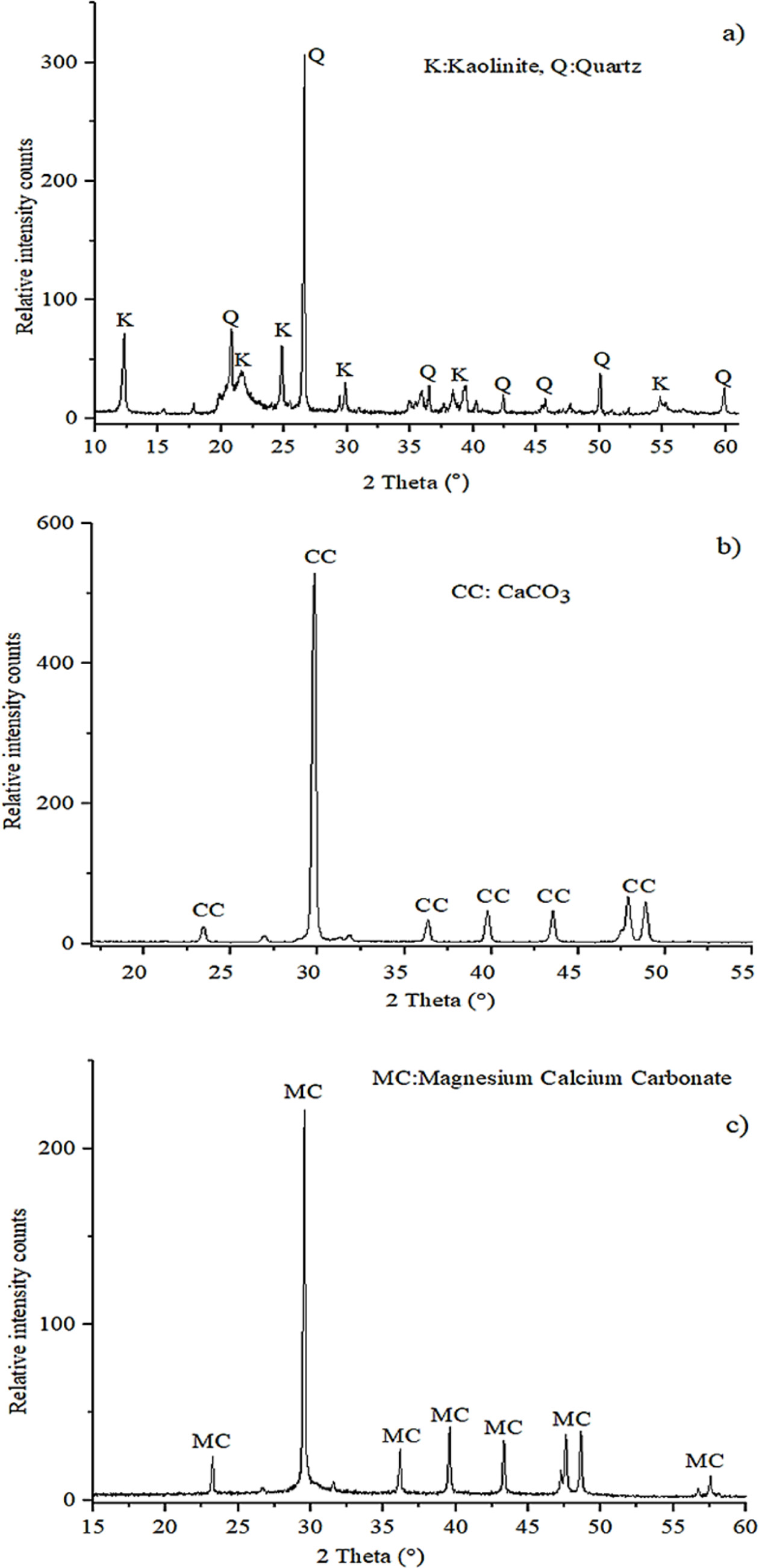
|
Fig. 1 XRD phase analysis of the raw materials: clay (a), calcite (b) and PFC waste (c). |
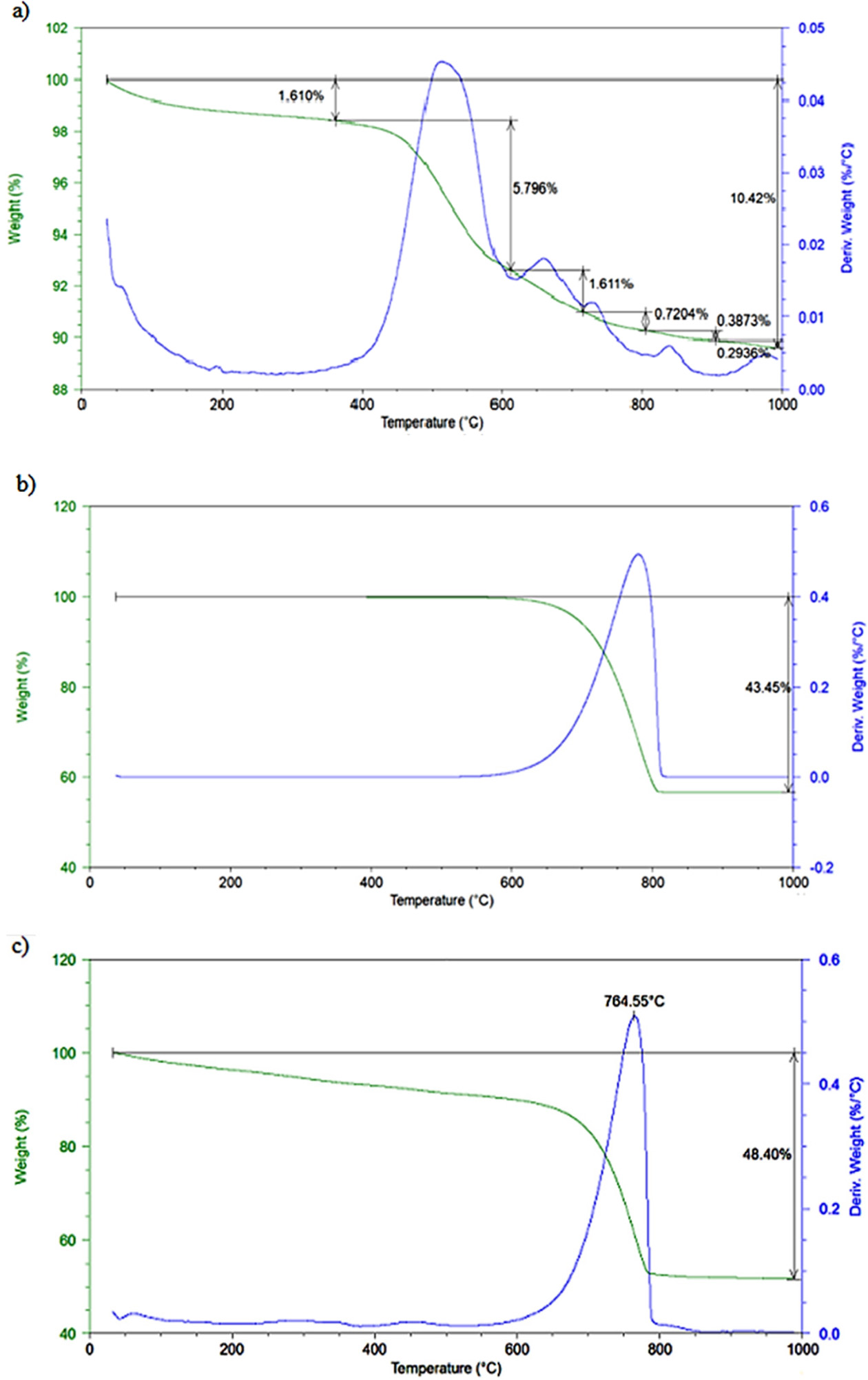
|
Fig. 2 TG analysis of the raw materials: (a) clay, (b) calcite and (c) PFC waste. |
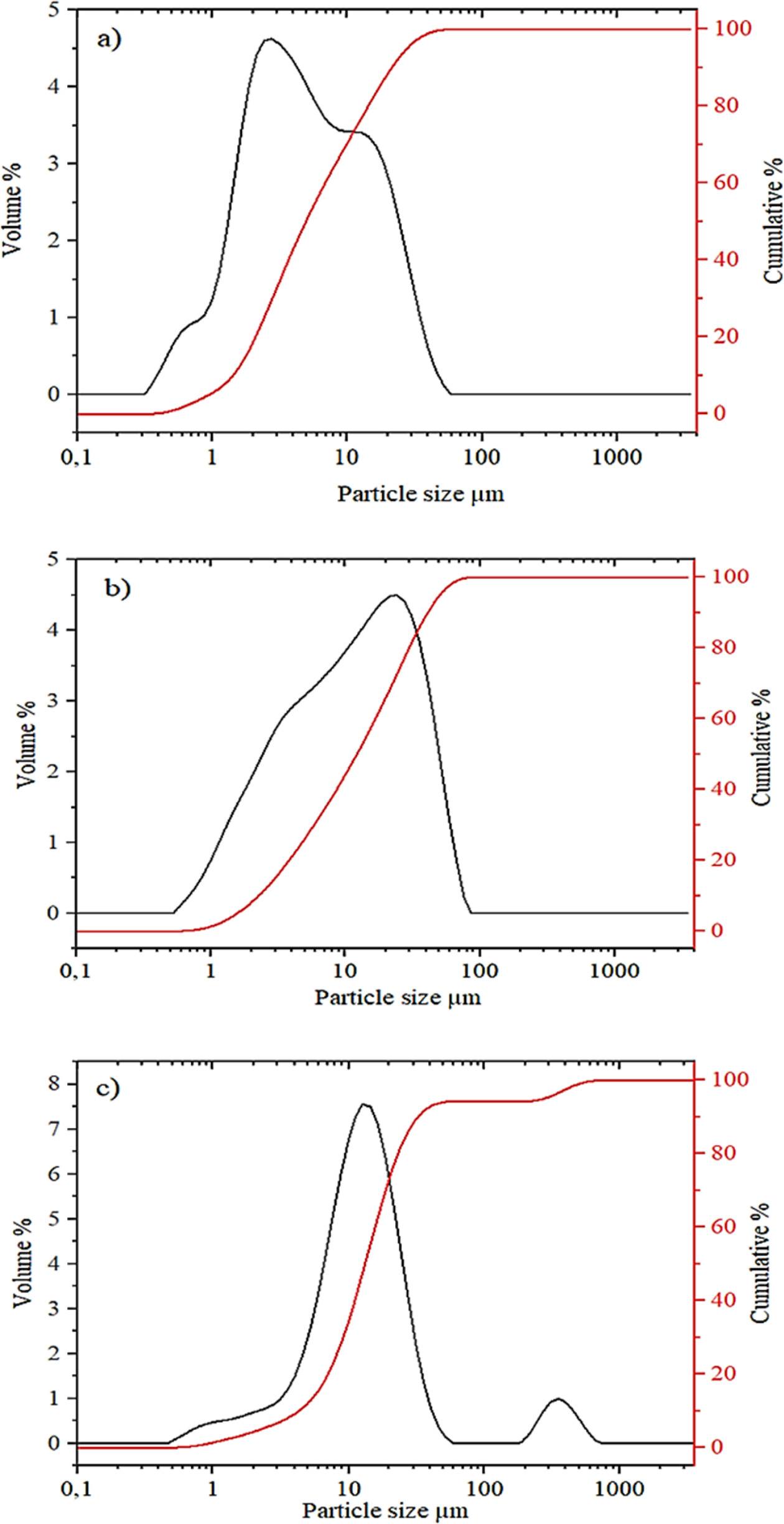
|
Fig. 3 Particle size analysis of the raw materials: clay (a), calcite (b) and PFC waste (c). |
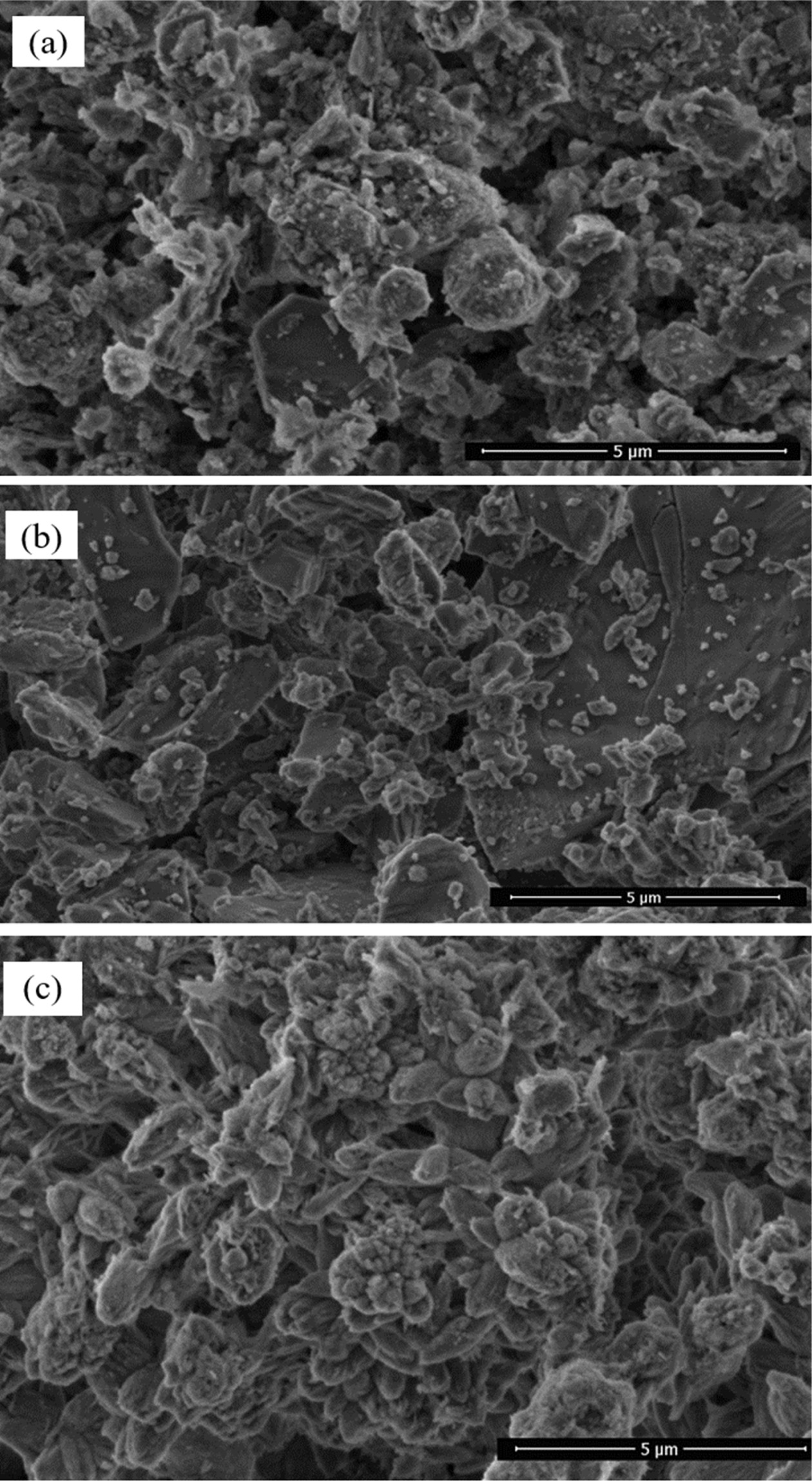
|
Fig. 4 SEM images of the raw materials: clay (a), calcite (b) and PFC waste (c). |
|
Fig. 5 Fired ceramic samples. |
|
Fig. 6 Phase analysis of CC samples fired in different temperatures (Q: Quartz-low, S: Cristobalite, W: Wollastonite, A: Anorthite, G: Gehlenite). |
|
Fig. 7 Phase analysis of CP samples fired in different temperatures (Q: Quartz-low, S: Cristobalite, W: Wollastonite, A: Anorthite, G: Gehlenite). |
|
Fig. 8 Compressive strengths of CP and CC samples fired at different temperatures. |
|
Fig. 9 Thermal conductivities of CP and CC samples fired at different temperatures. |
|
Fig. 10 SEM micrograph and EDS patterns of the CC samples: (a) fired at 1150 oC, (b) fired at 1200 oC. |
|
Fig. 11 SEM micrograph and EDS patterns of the CP samples: (a) fired at 1150 oC, (b) fired at 1200 oC. |
|
Table 2 Physical properties of CP and CC samples fired at different temperatures (LFS: linear firing shrinkage, WA: water absorption, AP: apparent porosity, BD: bulk density) |
The differences in CaO content between calcite and sugar
filter cake (PFC) waste, as well as organic content of PFC,
caused differences in physical, mechanical and microstructural properties. As
the result of higher loss of ignition of PFC waste, CP
(Clay + PFC waste) ceramics have higher porosity
percentages. As a function of high porosity, the bulk density, compressive
strength and thermal conductivity values of CP ceramics are lower than that of
CC (Clay + Calcite) ceramics. However, the dimensional stability of
CP samples at higher temperatures was found to be better than CC samples.
Depending on the firing temperature, the compressive strengths and apparent
porosity of fired samples produced from sugar filter cake waste ranged from 50.7
to 80.3 MPa and from 9.7% to 43.6%, respectively.
Phase analysis results show that due to lack of sufficient
CaO content in CP ceramics, the highest amount of cristobalite phase and
secondly anorthite are formed at 1200 oC. In contrast, CC
ceramics at the same temperature showed the strongest anorthite phase as
well as the glassy phase. In microstructural investiga- tions, it was observed that
porosity decreased and glassy phase increased by dissolving
silica with increasing temperature of samples. In
addition, calcium alumina silicate crystals appeared embedded in the glassy
matrix of CC samples. Sugar filter cake waste can be used instead of calcite in
the production of CAS ceramics, but due to the difference in CaO content, the
chemical composition of the desired phases in the ceramic body should be
considered. This study is important to show that this waste can be used in the
production of porous anorthite based ceramics. It is envisaged that in the next
process waste can be used as an alternative raw material to produce
thermal insulating materials with higher porosity.
Authors gratefully acknowledge the financial support with
project # 2018-TDR-FEBE-40 provided by Izmir Katip Çelebi University.
- 1. R.A. Gdula, Am. Ceram. Soc. Bull. 50 (1971) 555-557.
- 2. Y. Kobayashi and E. Kato, J. Am. Ceram. Soc. 77[3] (1994) 833-834.
-

- 3. A. Mergen and Z. Aslanoglu, Ceram. Int. 29 (2003) 667-670.
-

- 4. M.R. Boudchicha, S. Achour, and A. Harabi, J. Mater. Sci. Lett. 20 (2001) 215-217.
-

- 5. S. Kavalci, E. Yalamac, and S. Akkurt, Ceram. Int. 34 (2008) 1629-1635.
-

- 6. K. Okada, N. Watanabe, K.V. Jha, Y. Kameshima, A. Yasumori, and K.J.D. MacKenzie, Appl. Clay Sci. 23 (2003) 329-336.
-

- 7. K. Traoré, T.S. Kabré, and P.Blanchart, Ceram. Int. 29 (2003) 377-383.
-

- 8. S. Kurama and E. Ozel, Ceram. Int. 35 (2009) 827-830.
-

- 9. S. Dasgupta and S.K. Das, Bull. Mater. Sci., 25[5] (2002) 381-385.
-

- 10. M. Hojamberdiev, J.D. Torrey, M.S. Beltrã, and L. Wondraczek, J. Am. Ceram. Soc. 92[11] (2009) 2598-2604.
-

- 11. M. Sutcu and S. Akkurt, J. Eur. Ceram. Soc. 30[8] (2010) 1785-1793.
-

- 12. A.L. Yurkov and L.M. Aksel'rod, Refract. Ind. Ceram. 46[3] (2005) 170-174.
-

- 13. L.A. Dergaputskaya, A.N. Gaodu, and L.G. Litvin, Refract. Ind. Ceram. 21[7] (1980) 366-368.
-

- 14. J. Qin, C. Cui, X. Cui, A. Hussain, C. Yang, and S. Yang, Ceram. Int. 41[4] (2015) 5648-5655.
-

- 15. S.J. Lee and G.S. Kim, J. Ceram. Process. Res. 3[3] (2002) 136-140.
- 16. C.H. Lee, H.S. Shin, D.H. Yeo, H.T. Kim, and S. Nahm, J. Ceram. Process. Res. 16[3] (2015) 357-360.
- 17. S. Wang, X. Qi, J. Hu, and X. Tian, J. Ceram. Process. Res. 16[3] (2015) 361-365.
- 18. Z.B. Ozturk, J. Ceram. Process. Res. 17[6] (2016) 555-559.
- 19. H. Lu, M. He, Y. Liu, J. Guo, L. Zhang, D. Chen, H. Wang, H. Xu, and R. Zhang, J. Ceram. Process. Res. 12[5] (2011) 588-591
- 20. C. Li, C. Bian, Y. Han, C.A. Wang, and L. An, J. Eur. Ceram. Soc. 36[3] (2016) 761-765.
-

- 21. H.F. El-Maghraby, A.A. Aly, and S.M. Naga, Interceram-International Ceramic Review, 62 (2013) 426-428.
- 22. S.M. Naga, M. Sayed, H.F. Elmaghraby, M.S. Khalil, and M.A. El-Sayed, Ceram. Int. 43[8] (2017) 6024-6028.
-

- 23. S.M. Naga, H.F. El-Maghraby, and A.A. Aly, Interceram-International Ceramic Review 64[1-2] (2015) 34-37.
-

- 24. J. Man, W. Gao, S. Yan, G. Liu, and H. Hao, Constr. Build. Mater. 156 (2017) 1035-1042.
-

- 25. TürkŞeker, in “Sektör Raporu 2018: Yerli Üretimde Milli Değ,” (TürkŞeker, 2019) p.1-59.
- 26. ASTM Standards, No. C20 (2005) p.3.
 This Article
This Article
-
2020; 21(4): 425-432
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.425
- Received on Jan 17, 2020
- Revised on Apr 30, 2020
- Accepted on Jun 10, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Mucahit Sutcu
-
Izmir Katip Çelebi University, Faculty of Engineering and Architecture, Department of Materials Science and Engineering, İzmir 35620, Turkey
Tel : +90232 3293535
Fax: +90232 3253360 - E-mail: mucahit.sutcu@ikcu.edu.tr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.