- Preparation and characterization of hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 by the solid-state reaction route
Yujie Yang*, Xiansong Liu, Shuangjiu Feng, Xucai Kan, Qingrong Lv, Feng Hu, Jiangli Ni, Chaocheng Liu and Wei Wang
Engineering Technology Research Center of Magnetic Materials, School of Physics & Materials Science, Anhui University, Hefei 230601, P. R. China
The microstructural, spectral, magnetic and electric properties of hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 (0.00 ¡Â x ¡Â 0.30) synthesized by the solid-state reaction route have been studied. XRD results confirmed that the hexaferrites with Nd-Zn content (x) of 0.00 ¡Â x ¡Â 0.24 were single M-type phase, and the hexaferrite with x = 0.30 exhibited the M-type phase and impurity phase. The remanence (Br) increased with x from 0.00 to 0.06, and then decreased when x ¡Ã 0.06. The intrinsic coercivity (Hcj) and magnetic induction coercivity (Hcb) decreased with x from 0.00 to 0.30. Br indicated a linear decreasing behavior with increasing temperature from 20 oC to 140 oC. Hcj raised linearly with increasing temperature from 20 oC to 140 oC. The value of aBr basically remained constant with Nd-Zn content (x). The value of aHcj increased with x from 0.00 to 0.12, and began to decrease when x ¡Ã 0.12. The electrical resitivity (¥ñ) presented a decreasing trend with x from 0.00 to 0.30.
Keywords: M-type hexaferrites, Solid-state reaction route, X-ray diffraction, Magnetic measurements
Hexagonal ferrites have received considerable attention
of researchers and engineers since the discovery of the ferrite in the 1950s.
Although not as powerful as the newest NdFeB or SmCo5 magnets, they
still have a large market share in the market of magnetic materials, because of
their perfect chemical stability, high Curie temperature, high
performance-price ratio, and easy methods of production [1, 2]. In addition,
M-type hexaferrites can be widely utilized as microwave devices,
permanent magnetic materials, radar communication and microwave
devices [3-5]. Many preparation techniques have been
used to prepare the M-type hexaferrites such as hydrothermal method [6],
three-step calcination method [7], co-precipitation method [8], standard
solid-state reaction route [9], molten flux calcination method [10], sol-gel
method [11], pulsed laser deposition method [12], and extrusion-based three-dimensional
(3D) printing [13]. In the above-mentioned methods, the solid-state reaction
route was used to fabricate the hexaferrites because of a few several
profitable factors, for example controllable grain size, low manufacturing
cost, simple technology and high productive.
In order to modify the intrinsic magnetic properties of
M-type hexaferrites, the M-type hexaferrites been doped with rare-earth metals
and transition metals, or the combination of these [14-36]. Singh et al.
reported the rare-earth substitution (La3+, Nd3+ and Sm3+
) doped strontium ferrite (Sr-M), and the results exhibited that the
magnetization moment (Ms) and remenance (Mr)
decrease with increasing rare-earth ions substitution, while the
enhancement of Hc values may be due to higher
magnetocrystalline anisotropy [15]. The Nd-substituted strontium
hexaferrites prepared by hydrothermal synthesis have been
reported by Wang et al. [17], and it is found that Nd substitution with a Nd-Sr
ratio of 1/8 enhances the coercivity without causing any
significant deterioration in either the saturation
magnetization or the remanence. Shekhawat et al. synthesized the La-Sm
substituted Sr-hexaferrite SrAl4(La0.5Sm0.5)xFe8-xO19
(0.0 ≤ x ≤ 1.5) nanomaterials by the auto combustion method and found
that the remanence magnetization increases while intrinsic
coercivity decreases with substitution [18]. The Zn-doped Ba hexaferrite single
crystals have been synthesized, and it is found that Zn substitution significantly
influences the coercivity and magnetization of Ba
hexaferrite while the Curie temperature was nearly constant over the range of
doping [23]. Zhang et al. reported the Nd-Co doped strontium hexaferrites Sr1-xNdxFe12-xCoxO19
(0.0 ≤ x ≤ 0.4) fabricated by sol-gel autocombustion method, and the
results showed that Nd-Co substitution can improve the saturation magnetization
and coercivity and reaches a maximum at x=0.2 [33]. Liu et al.
prepared the Ce-Zn co-substituted M-type strontium hexaferrites by the
ceramic method, and the results showed that Ms and Hc
can be improved significantly [36].
In this article, in order to enhance
the magnetic properties, we have selected a combination of Nd3+ and
Zn2+ ions to substitute in the M-type Ba-Sr hexaferrites. The
hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
(0.00 ¡Â x ¡Â 0.30) were successfully synthesized by the
solid-state reaction route. Impact of Nd-Zn co-doping on the
microstructural, spectral and electric properties was analyzed. Subsequently,
the magnetic properties of hexaferrites with different Nd-Zn content
(x) were systematically investigated under
different temperatures from room
temperature (20 oC) to 140 oC. The novelty of this work is doing a study on the temperature coefficient of remanence (Br) (aBr), and
temperature coefficient of intrinsic coercivity (Hcj) (aHcj) for the Nd-Zn co-doped hexaferrites.
The hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
(0.00 ≤ x ≤ 0.30) were prepared via the solid-state reaction route
[37]. Barium carbonate (BaCO3), strontium carbonate
(SrCO3), metallic oxides (Nd2O3, Fe2O3
and ZnO) were weighted in stoichimetric ratio. And then, the raw materials were
thoroughly mixed together in water for 10 h in a ball mill in order to obtain
finely mixed powder. Further, the as-mixed powder was dried, and pressed into
circular pellets with Φ30 × 16 mm. Subsequently, the pellets
were calcined in a muffle furnace at 1,260 oC for
2.0 h. The calcined pellets were shattered in a vibration mill, and suitable
additives (CaCO3 0.8 wt%, SiO2 0.2 wt% and Al2O3
0.2 wt%) were added, then the mixture was wet-milled in a ball-mill for
16 h. This procedure guarantees a narrow particle size distribution with the
mean size of around 0.75 μm. The fine milled hexaferrite slurry was pressed
into circular pellets with Φ30 × 16 mm in a magnetic field of 1.2 T. In
order to measure the DC electrical resitivity, the fine milled hexaferrite
slurry was dried, and then pressed into circular pellets with Φ20 × 8
mm. Finally, all green pellets were sintered in a muffle furnace at 1,185 oC
for 1.5 h.
The phase identification and structural characterization of
synthesized samples were performed by X-ray diffraction (XRD,
Rigaku Smartlab). The surface morphology of the hexaferrites was detected by a
field emission scanning electron microscopy (FE-SEM, HITACHI S-4800). The
magnetic properties were measured at different temperatures from room
temperature (20 oC) to 140 oC
using a Hysteresis graph meter (NIM-2000HF, National
Institute of Metrology of China). (Resistivity testing system, Ningbo rooko
FT-353) was used to measure the DC electrical resitivity (ρ) of
the hexaferrites at room temperature.
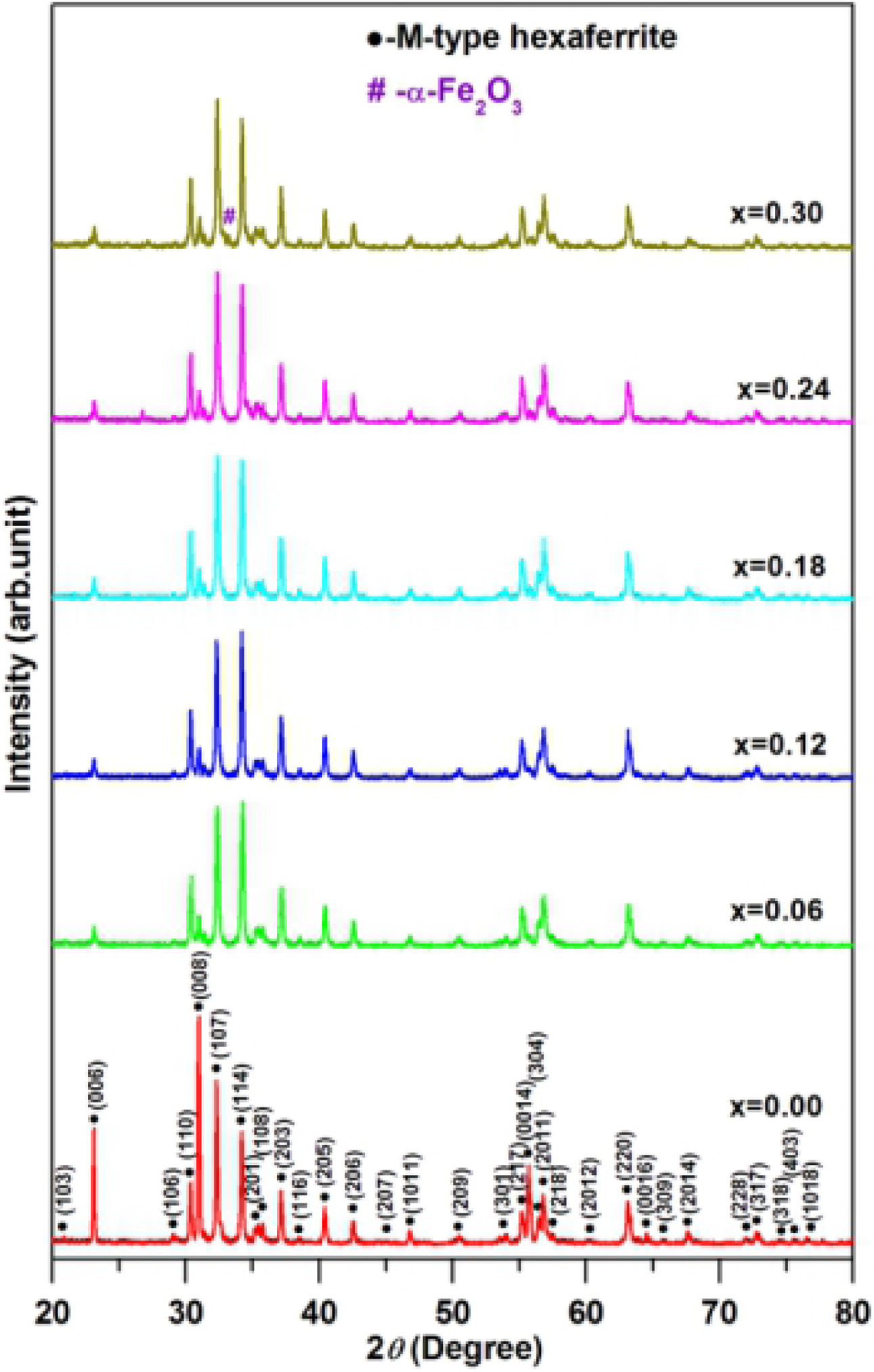
Fig. 1 presents the XRD patterns of hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x). The characteristics
peaks were compared with the M-type hexaferrite ICCD card No.
51-1879. It can be observed that the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-x ZnxO19 with
Nd-Zn content (x) ≤ 0.24 belonged to M-type hexagonal crystal structure. The
presence of hematite (α-Fe2O3) (ICCD card no. 89-0599)
detected for the hexaferrite with Nd-Zn content (x) = 0.30 might be due the
incomplete reaction under the preparing conditions. These show that the maximum
doped content of Nd-Zn co-doping can not exceed x = 0.24.
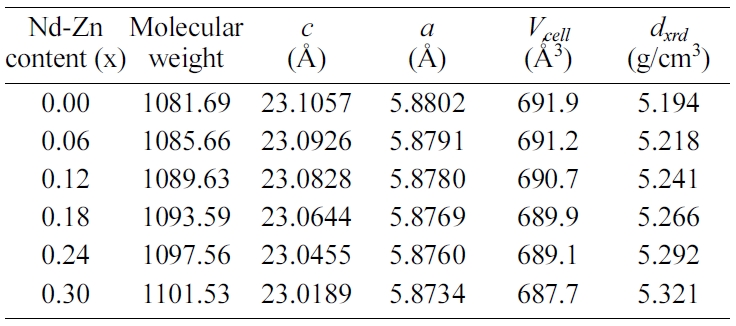
The lattice parameters c and a of the
hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
were calculated using to the following formula [38].
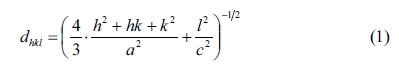
In the given relation, dhkl is the
interplaner spacing of the lines in XRD pattern and h, k and l
are the Miller indices. The values of lattice parameters c and a
for the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x) are listed in Table 1. The values
of c and a decrease with increasing Nd-Zn content (x) from 0.00
to 0.30. The possible explanation for the decrease of c and a
with increasing Nd-Zn content (x) can be attributed to the difference in the
ionic radii (Δr) of the metal ions and the number of ionic substitutions
of each species. Substitution of Sr2+ (r = 1.180 Å) by Nd3+
(r = 0.983 Å) makes a negative difference in the ionic radii of Δr
= -0.197 Å. Substitution of Fe3+ (r = 0.645 Å) by Zn2+ (r
= 0. 740 Å) makes a positive difference in the ionic radii of Δr =
+0.095 Å. As in the case of Nd-Zn content (x) = 0.12, the lattice parameter
c and a are decreased.
The unit cell volume (Vcell) of the
hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
was calculated from the following equation [38]:

And its values are summarized in Table 1. It is clearly
seen that the unit cell volume (Vcell) decreases with Nd-Zn
content (x) from 0.00 to 0.30. The decrease in the Vcell is
due to the decrease of lattice parameters c and a with increasing
Nd-Zn content (x) as shown in Table 1. The X-ray density (dxrd)
has been calculated by using the below formula [39]:

where Z is the number of
molecular per unit cell, M is the molecular weight, NA
is Avogadro’s number and Vcell is unit cell volume. And the
values dxrd are listed in Table 1. It noticed that the value
of dX-ray enhances from 5.194 g/cm3 at x = 0.00 to
5.321 g/cm3 at x = 0.30. The enhancement of dxrd
with increasing Nd-Zn content (x) can be attributed to the larger molecular
weight and decreasing trend of unit cell volume (Vcell) for the Nd-Zn co-doped
hexaferrites displayed in Table 1.
Fig. 2 shows the FE-SEM images of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with x = 0.00, x = 0.12, and x = 0.24. This
indicates that the grains in the hexaferrites are homogeneous
distribution with hexagonal plate like shape. The average grain
size increases from 1.8 μm at x = 0.00 to 3.2 μm at
x = 0.24.
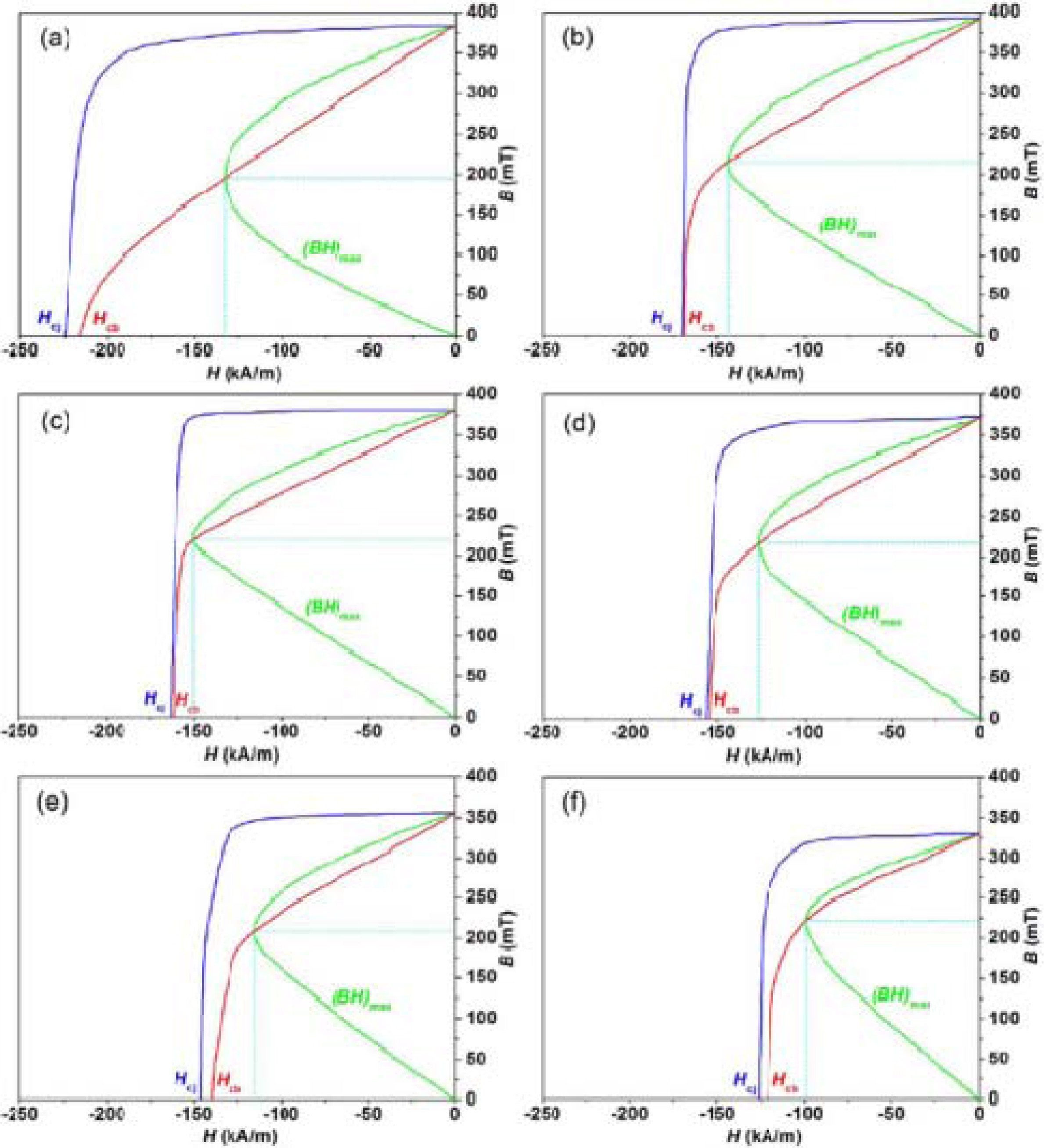
Fig. 3 represents the room temperature demagnetizing curves
of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x). As revealed by the demagnetizing curves, the
magnetic properties of the hexaferrites are greatly influenced by the
substitution of Sr2+ ion by Nd3+ ion and Fe3+
ion by Zn2+ ion. The remanence (Br), magnetic
induction coercivity (Hcb), and intrinsic coercivity (Hcj)
at the room temperature are derived from the demagnetizing curves of the
hexaferrites samples with different Nd-Zn content (x).
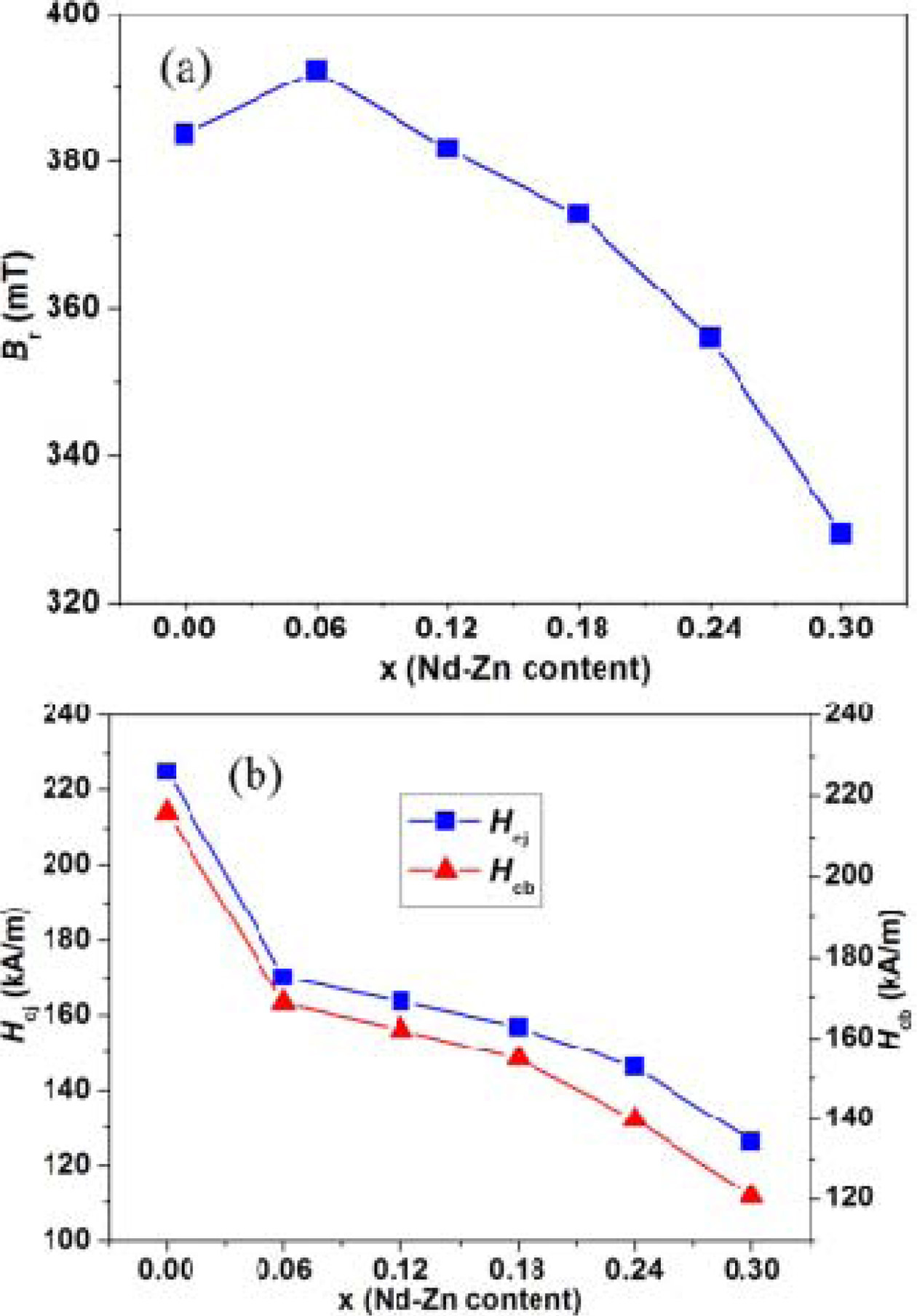
The remanence (Br) of the hexaferrites
Ba0.40Sr0.60-x NdxFe12.00-xZnxO19
as a function of Nd-Zn content (x) is plotted in Fig. 4(a). It is clearer
that a suitable amount of Nd-Zn substitution can increase the remanence (Br)
of the hexaferrites. In Fig. 4(a), with increasing Nd-Zn content
(x), Br first increases and reaches to the maximum
value of 386.0 mT at Nd-Zn content (x) = 0.06, and then decreases when Nd-Zn
content (x) ≥ 0.06. The changing trend of remanence (Br)
can be explained on the basis of the occupation of the substituted ions and the
impurity phase. M-type hexaferrites belong to P63/mmc space group,
and have five crystallographic sublattices, such as three kinds of octahedral
sites (2a, 12k, 4f2), one tetrahedral site (4f1)
and one bipyramidal site (2b). The 2a, 12k and 2b
sites have upward spin direction, and the 4f1 and 4f2
sites have downward spin direction [37, 40]. Herme et al. [41]
have reported that in the M-type hexaferrites, Nd3+ ions substitute
the Sr2+ sites in the vicinity of 4f2 sites via
Mössbauer analysis. Lee et al. [42] have reported that Zn2+ ions
prefer to occupy the 4f1 and 2b sites of tetrahedral
sites in the M-type hexagonal structure by Mössbauer spectra. Zn2+ ion
is nonmagnetic. And the magnetic moment of Fe3+ ion is 5 μB.
Therefore, the increase of remanence (Br) for the
hexaferrites with increasing Nd-Zn content (x) from 0.00 to 0.06 is probably
due to the below factor. When Nd-Zn content (x) ≤ 0.06, the number of Zn2+
ions entering 4f1 sites (spin down) is
more than that of Zn2+ ions entering 2b sites (spin
up). This in turn decreases the negative magnetic moment of Fe3+ ions.
Consequently, according to equation (3), the whole magnetic
moment is enhanced. This leads to the increase of the
remanence (Br). When Nd-Zn content (x) ≥ 0.06, the
decrease of remanence (Br) for the hexaferrites may be
attributed to the below three reasons. Firstly, at Nd-Zn content (x) ≥ 0.06, Zn2+
ions substitute Fe3+ ions in 2b sites having spin-up.
According to equation (3), this in turn decreases the whole magnetic moment.
This results in the decrease of the remanence (Br).
Secondly, Zn2+ (0 μB) ions substituting magnetic Fe3+
ions (5 μB) weaken the super-exchange interaction
between metallic ions in the M-type hexaferrites, and then the
remanence (Br) is decreased. Thirdly, when Nd-Zn
content (x) = 0.30, as seen from Fig. 1, the impurity phase
(hematite: α-Fe2O3) leads to the decrease of
remanence (Br).
Fig. 4(b) shows the intrinsic coercivity (Hcj)
and magnetic induction coercivity (Hcb) of the hexaferrites
Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
as a function of Nd-Zn content (x). As shown in Fig. 4(b), the values of Hcj
and Hcb show a decreasing trend with increasing Nd-Zn content
(x), and decrease from 225.0 and 215.9 kA/m at x = 0.00 to 126.2 and 120.7 kA/m
at x = 0.30, respectively. This proposes that Nd-Zn co-substitution can modify
the coercivity of M-type Ba-Sr hexaferrites. Yang et al. [43] have
reported that in the M-type hexaferrites, the ions at octahedral
4f2 site and bipyramidal 2b
site are known to be the main contributors to the magnetocrystalline
anisotropy. According to the Stroner-Wohlfarth theory, the
intrinsic coercivity (Hcj) of the M-type
hexaferrites which results from coherent rotation of
magnetization can be calculated by the following equation [44]:
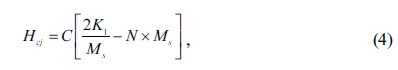
where C is dimensionless constant of
material, K1 is the first anisotropy constant, and
N is the grain demagnetization factor. Therefore, according
to the equation (4), the decrease of Hcj with increasing
Nd-Zn content (x) from 0.00 to 0.30 could be due to the following two reasons.
Firstly, as seen from Fig. 2, the average grain size of M-type hexaferrite
increases with increasing Nd-Zn content (x) from 0.00 to 0.30. Thus, the value
of N increases with increasing Nd-Zn content (x). According to equation
(4), the value of Hcj is decreased. Secondly, the results of
Mössbauer spectra showed that Zn2+ ions prefer to occupy the 4f1
and 2b sites of tetrahedral sites in the M-type hexagonal structure
[42]. The decrease of Hcj with increasing Nd-Zn content (x)
may be related to the reduction of magnetocrystalline anisotropy
field as a result of Zn2+ substitution for Fe3+ ions at 2b
sites.
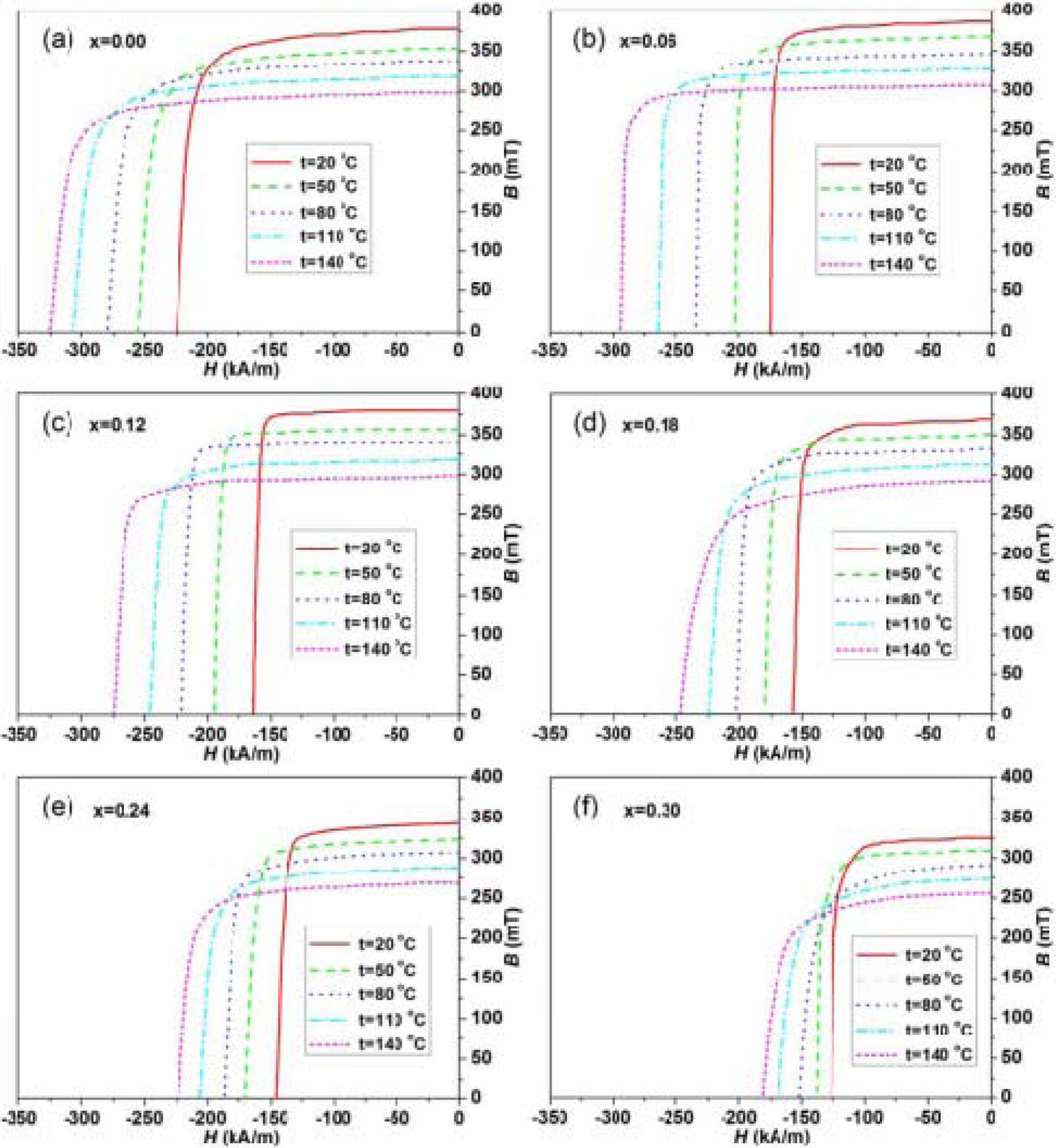
The temperature dependent demagnetizing curves of the
hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x) are presented in Fig. 5. The changing trend of
the demagnetizing curves measured at different temperatures is in agreement
with that reported by T.D.K. Corporation [45] and A. Goldman et al. [46]. The
values of the remanence (Br), and intrinsic coercivity (Hcj)
at different temperatures are calculated from the demagnetizing curves for the
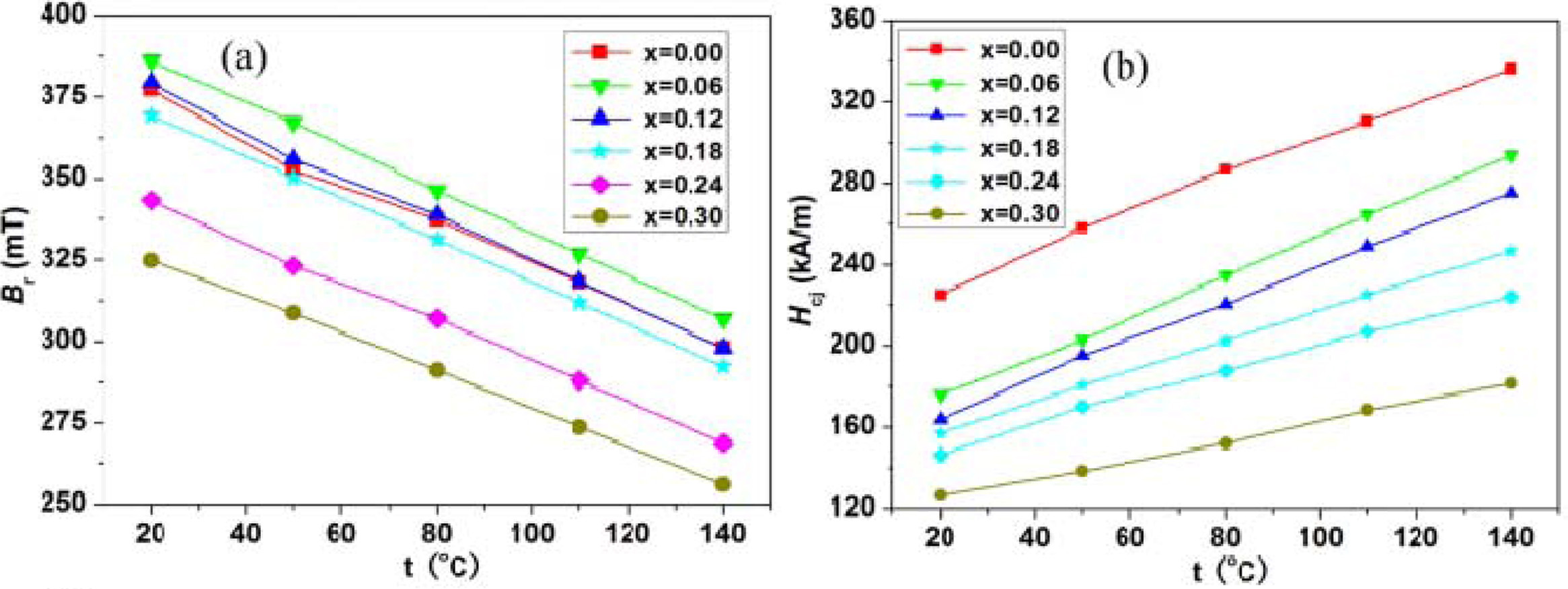
hexaferrites with different Nd-Zn content (x). Fig. 6(a) presents the
temperature dependent remanence (Br) between 20 oC
and 140 oC for the hexaferrites with different Nd-Zn content (x). As
seen from Fig. 6(a), for the hexaferrites with different Nd-Zn content (x), the
values of remanence (Br) have a linear decreasing behavior
with increasing temperature from 20 oC to 140 oC.
This is in agreement with the results reported by Zhou et al. [47]. The
variations of the intrinsic coercivity (Hcj) between 20 oC
and 140 oC for the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x) are shown in Fig.
6(b). It is observed that for the hexaferrites with different Nd-Zn
content (x), the values of intrinsic coercivity (Hcj) raise
linearly with increasing temperature from 20 oC to 140 oC.
This agrees with the changing trend reported by W. Zhou [48] and T.D.K. Corporation
[49].
When the ambient temperature ¡Â 400
oC, the remanence (Br)
of hexaferrites BaFe12O19 deareases linearly with increasing temperature [50]. The temperature
coefficient of remanence (Br)
can be approximated as a constant in a certain temperature range. Therefore,
the average temperature coefficient of Br (aBr) of
hexaferrites can be defined as the below relation [48]:
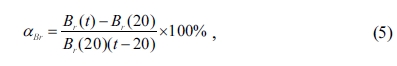
where Br(20)
is the value of remanence (Br) measured at 20 oC,
Br(t) is the value of remanence (Br)
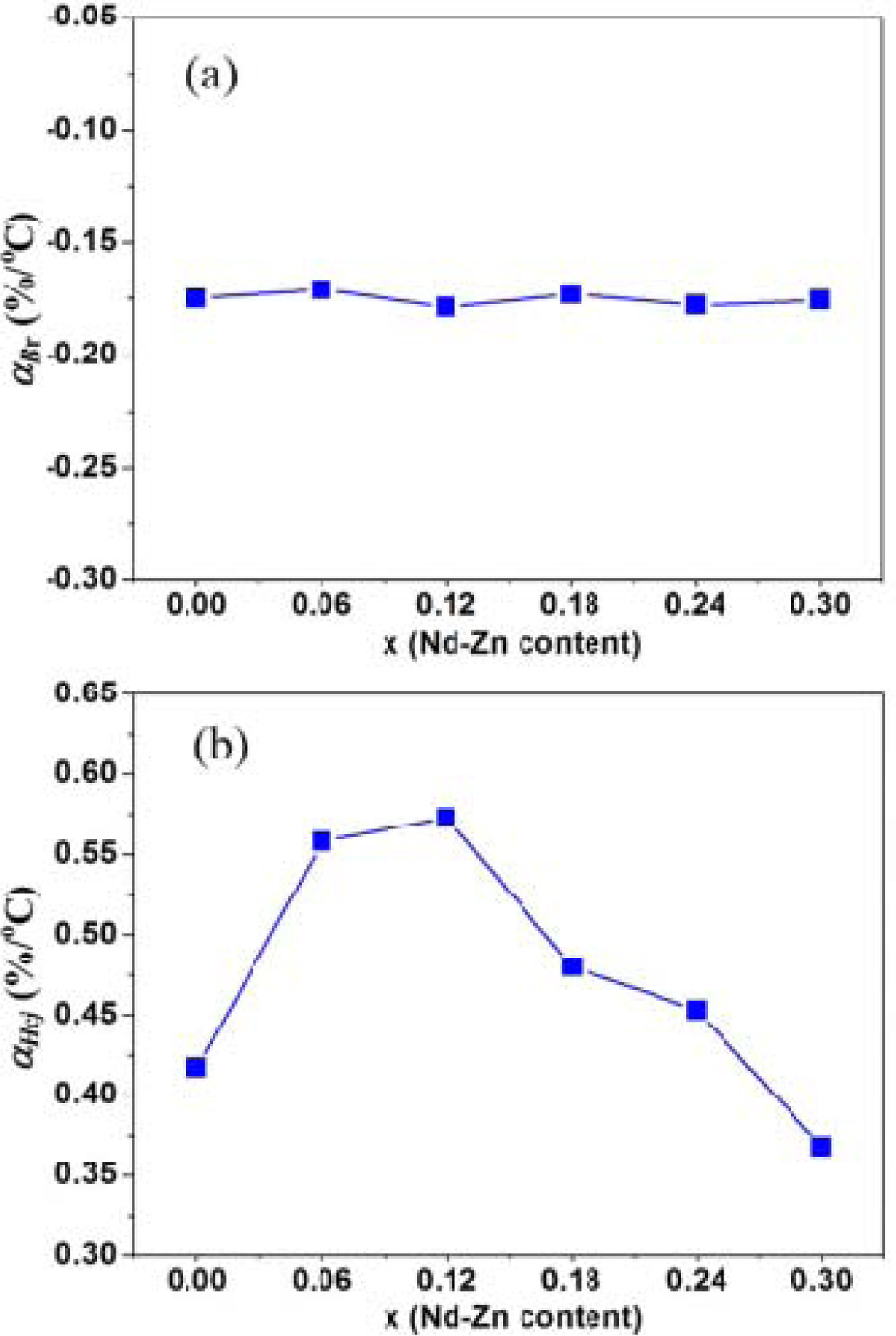
measured at temperature of t oC. Fig. 7(a) represents the variation
of aBr of
the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
as a function of Nd-Zn content (x). It is clear from Fig. 7(a) that the
value of aBr basically remains constant around -0.175 %/oC.
The value of aBr is in agreement with that reported by T.D.K.
Corporation [45]. This indicates
that the Nd-Zn substitution have not big impact on the average temperature coefficient of remanence
(Br) (aBr) of the
hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19.
As shown in Fig. 6(b), the values of
intrinsic coercivity (Hcj)
have a linear behavior with temperature from 20 oC to 140 oC.
The temperature coefficient of intrinsic coercivity (Hcj) is
also approximated as a constant in a certain temperature range [49]. Thus, the
average temperature coefficient of Hcj (aHcj) can be calculated using the following equation [43]:
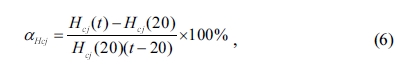
where Hcj(20)
is the value of intrinsic coercivity (Hcj) measured at 20 oC,
Hcj(t) is the value of intrinsic coercivity (Hcj)
measured at temperature of t oC. The influence of Nd-Zn content (x) on the average temperature coefficient of intrinsic coercivity (Hcj)
(aHcj) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
is plotted in Fig. 7(b). It is noted that the value of aHcj firstly increases from 0.417 %/oC at x=0.00 to
0.573 %/oC at x = 0.12, and then decreases when Nd-Zn content (x) ¡Ã
0.12. This shows that Nd-Zn content
(x) can significantly affect the
values of aHcj.
The values of aHcj are larger
than that reported by T.D.K. Corporation [49], which should the values of intrinsic coercivity (Hcj)
in this study are lower than that in TDK Products Catalog [49].
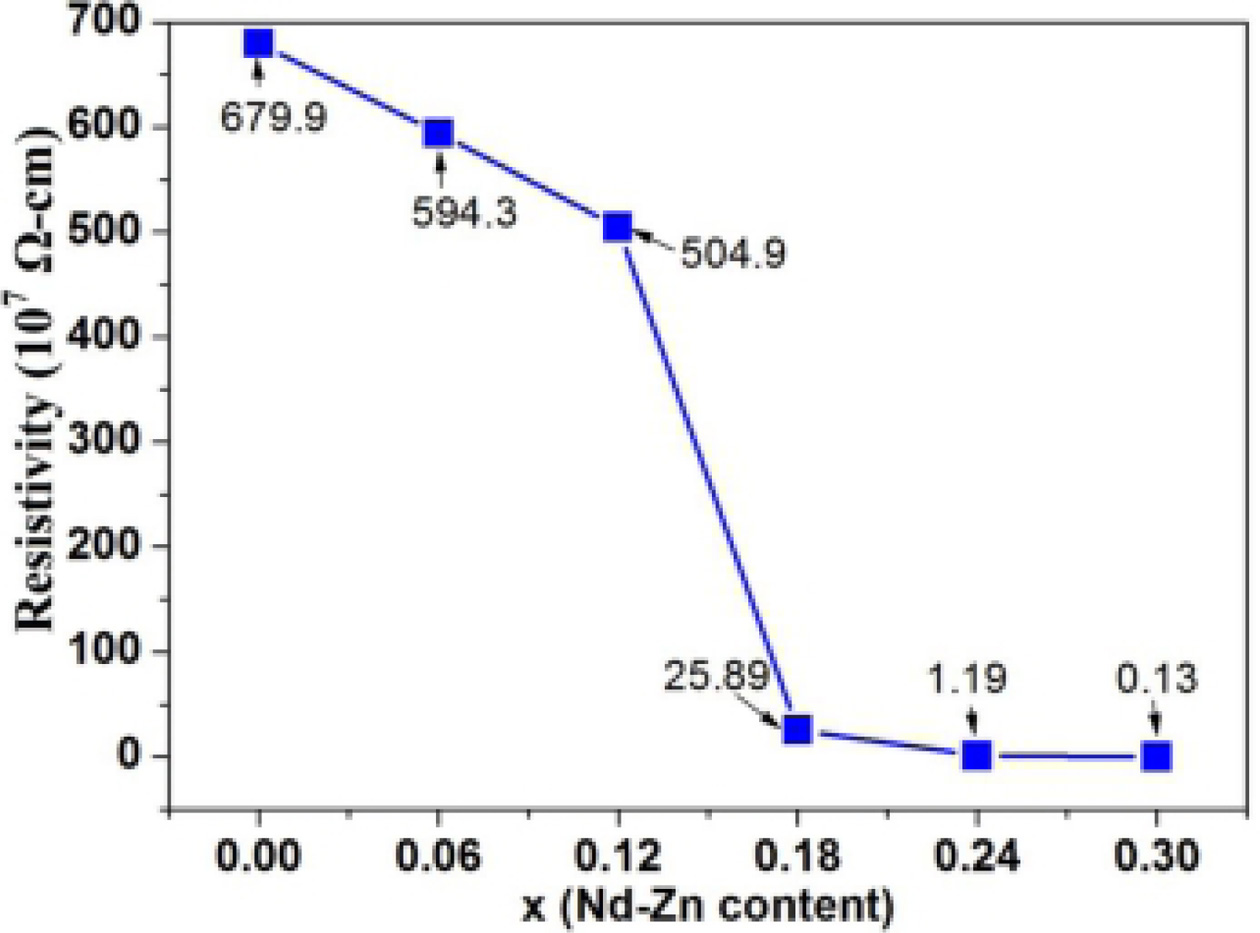
Fig. 8 illustrates the impact of Nd-Zn content (x) on the
DC electrical resistivity (ρ) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19.
It is clear that the electrical resistivity (ρ)
is strongly affected by Nd-Zn content (x). It is observed that the
value of electrical resitivity (ρ) decreases from 679.9 × 107
Ω-Cm at x = 0.00 to 0.13 × 107 Ω-Cm at x = 0.30. The
mechanism of conductivity in the hexaferrites is attributed to the hopping of
electrons between Fe3+ and Fe2+ ions at the octahedral
sites [50]. Van Diepen et al. have reported that the substitution of La3+
for Ba2+ or Sr2+ in M-type ferrites is associated with a
valence change of Fe3+ to Fe2+ at 2a or 4f2
site [51]. Thus, as Nd3+ ions substitute Sr2+ ions and Zn2+
ions substitute Fe3+ ions, some Fe3+ ions
will change into Fe2+ ions. This increases the number
of Fe2+ ions which leads to the increase of the hopping probability
between the Fe3+ and Fe2+ ions. Thus, the above reasons
result in the decrease of electrical resitivity (ρ).
|
Fig. 1 XRD patterns of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 with different Nd-Zn content (x). |

|
Fig. 2 FE-SEM images of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 with (a) x=0.00, (b) x=0.12, and (c) x=0.24. |
|
Fig. 3 Room temperature demagnetizing curves of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 with (a) x = 0.00, (b) x = 0.06, (c) x = 0.12, (d) x = 0.18, (e) x = 0.24, and (f) x = 0.30. |
|
Fig. 4 (a) Remanence (Br), and (b) Intrinsic coercivity (Hcj) and magnetic induction coercivity (Hcb) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 as a function of Nd-Zn content (x). |
|
Fig. 5 Measuring temperature dependent demagnetizing curves of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 with (a) x=0.00, (b) x=0.06, (c) x=0.12, (d) x=0.18, (e) x=0.24, and (f) x=0.30. |
|
Fig. 6 (a) The temperature dependent remanence (Br), and (b) The temperature dependent intrinsic coercivity (Hcj) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 with different Nd-Zn content (x) from x=0.00 to x=0.30, obtained from demagnetizing curves. |
|
Fig. 7 (a) The average temperature coefficient of remanence (Br) (aBr), and (b) The average temperature coefficient of intrinsic coercivity (Hcj) (aHcj) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 as a function of Nd-Zn content (x). |
|
Fig. 8 DC electrical resistivity (ρ) of the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19 as a function of Nd-Zn content (x). |
|
Table 1 The values of molecular
weight, lattice parameters (c and a), unit cell volume (Vcell),
X-ray density (dxrd), and bulk density (dbulk)
for the hexaferrites Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
with different Nd-Zn content (x). |
The solid-state reaction route was
used to synthesize the Nd-Zn substituted
M-type hexaferrites with nominal
compositions Ba0.40Sr0.60-xNdxFe12.00-xZnxO19
(0.00 ¡Â x ¡Â 0.30). The X-ray diffraction patterns show that the hexaferrites
with Nd-Zn content (x) of 0.00 ¡Â x ¡Â 0.24 were single M-type phase, while the
hexaferrites with Nd-Zn content (x) = 0.30 exhibited the M-type phase and
impurity phases. FE-SEM images proposed that all the particles were regular
hexagonal platelet-like shape and the average particle size increased with
increasing Nd-Zn content (x). Br increased with Nd-Zn content
(x) from 0.00 to 0.06, and then started to decrease when Nd-Zn content (x) ¡Ã
0.06. Hcj and Hcb decreased with Nd-Zn
content (x) from 0.00 to 0.30. Br indicated a linear
decreasing behavior with increasing temperature from 20 oC to
140 oC. Hcj raise linearly with increasing
temperature from 20 oC to 140 oC. The value of aBr basically
remained constant with Nd-Zn content (x). The value of aHcj firstly increased with x from 0.00 to 0.12, and then
decreased when x ¡Ã 0.12. The electrical resitivity (¥ñ) presented a
decreasing trend with x from 0.00 to 0.30.
This work was supported by the National Natural Science
Foundation of China (Nos. 51872004, 51802002), Education
Department of Anhui Province (Nos. KJ2013B293, KJ2018A0039), Key Program of the Science
and Technology of Anhui Province (Grant No. S201904a09020074).
- 1. S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, An.V. Trukhanov, E.L. Trukhanova, D.I. Tishkevich, V.M. Ivanov, T.I. Zubar, M. Salem, V.G. Kostishyn, L.V. Panina, D.A. Vinnik, S.A. Gudkova, Ceram. Int. 44 (2018) 290-300.
-

- 2. L.A. Trusov, E.A. Gorbachev, V.A. Lebedev, A.E. Sleptsova, I.V. Roslyakov, E.S. Kozlyakova, A.V. Vasiliev, R.E. Dinnebier, M. Jansen, P.E. Kazin, Chem. Commun. 54 (2018) 479-482.
-

- 3. T.T. Loan, T.T.V. NGA, N.P. Duong, S. Soontarnon, T.D. Hien, J. Electron. Mater. 46 (2017) 3396-3405.
-

- 4. I. Ali, M.U. Islam, M.S. Awan, M. Ahmad, J. Electron. Mater. 43 (2014) 512-521.
-

- 5. R. Valenzuela, in “Magnetic ceramics” (Cambridge University Press, 1994) p.191.
-

- 6. D. Makovec, D. Primc, S. Šturm, A. Kodre, D. Hanžel, M. Drofenik, J. Solid State Chem. 196 (2012) 63-71.
-

- 7. S. Mahadevan, S.B. Narang, P. Sharma, Ceram. Int. 45 (2019) 9000-9006.
-

- 8. N. Tran, H.S. Kim, T.L. Phan, D.S. Yang, B.W. Lee, Ceram. Int. 44 (2018) 12132-12136.
-

- 9. J. Dho, E.K. Lee, J.Y. Park, N.H. Hur, J. Magn. Magn. Mater. 285 (2005) 164-168.
- 10. C. Serletis, G. Litsardakis, E. Pavidou, K.G. Efthimiaadis, Physica B. 525 (2017) 78-83.
-

- 11. Z.H. Hua, S.Z. Li, Z.D. Han, D.H. Wang, M. Lu, W. Zhong, B.X. Gu, Y.W. Du, Mater. Sci. Eng. A 448 (2007) 326-329.
-

- 12. J.E. Beevers, C.L. Love, V.K. Lazarov, S.A. Cavill, H. Izadkhah, C. Vittoria, R. Fan, G. van der Laan, S.S. Dhesi, App. Phys. Lett. 112 (2016) 082401.
-

- 13. X.X. Wei, Y.H. Liu, D.J. Zhao, X.W. Mao, W.Y. Jiang, S.Z. Sam Ge, J. Magn. Magn. Mater. 493 (2020) 165664.
-

- 14. J.F. Wang, C.B. Ponton, I.R. Harris, J. Alloys Compd. 403 (2005) 104-109.
-

- 15. A. Singh, S.B. Narang, K. Singh, O.P. Pandey, R.K. Kotnala, J. Ceram. Process. Res. 11[2] (2010) 241–249.
-

- 16. A. Thakur, R.R. Singh, P.B. Barman, J. Magn. Magn. Mater. 326 (2013) 35-40.
-

- 17. J.F. Wang, C.B. Ponton, I.R. Harris, IEEE Trans. Magn. 38 (2002) 2928-2930.
-

- 18. D. Shekhawat, A.K. Singh, P.K. Roy, J. Mol. Struct. 1179 (2019) 787-794.
-

- 19. Y.J. Yang, J.X. Shao, F.H. Wang, D.H. Huang, J. Ceram. Process. Res. 18[5] (2017) 394–398.
- 20. J.F. Wang, C.B. Ponton, I.R. Harris, J. Magn. Magn. Mater. 298 (2006) 122-131.
-

- 21. P.A. Pawar, S.S. Desai, Q.Y. Tamboli, S.E. Shirsath, S.M. Patange, J. Magn. Magn. Mater. 378 (2015) 59-63.
-

- 22. G. Litsardakis, I. Manolakis, C. Serletis, K.G. Efthimiadis, J. Magn. Magn. Mater. 316 (2007) 170-173.
-

- 23. D.A. Vinnik, A.S. Semisalova, L.S. Mashkovtseva, A.K. Yakushechkina, S. Nemrava, S.A. Gudkova, D.A. Zherebtsov, N.S. Perov, L.I. Isaenko, R. Niewa, Mater. Chem. Phys. 163 (2015) 416-420.
-

- 24. X.-S. Liu, L. Fetnandez-Garcia, F. Hu, D.-R. Zhu, M. Suárez, J.L. Menéndez, Mater. Chem. Phys. 133 (2012) 961-964.
-

- 25. M. Ghimire, S. Yoon, L. Wang, D. Neupane, J. Alam, S.R. Mishra, J. Magn. Magn. Mater. 454 (2018) 110-120.
-

- 26. D.A. Vinnik, A.Yu. Tarasova, D.A. Zherebtsov, L.S. Mashkovtseva, S.A. Gudkova, S. Nemrava, A.K. Yakushechkina, A.S. Semisalova, L.I. Isaenko, R. Niewa, Ceram. Int. 41 (2015) 9172-9176.
-

- 27. J. Qiu, Y. Wang, M. Gu, Mater. Lett. 60 (2006) 2728-2732.
-

- 28. I.A. Auwal, H. Güngüneş, A. Baykal, S. Güner, S.E. Shirsath, M. Sertkol, Ceram. Int. 42 (2016) 8627-8635.
-

- 29. A. Shayan, M. Abdellahi, F. Shahmohammadian, S. Jabbarzare, A. Khandan, H. Ghayour, J. Alloys Compd. 708 (2017) 538-546.
-

- 30. H.M. Khan, M.U. Islam, Y.B. Xu, M.N. Ashiq, I. Ali, M.A. Iqbal, M. Ishaque, Ceram. Int. 40 (2014) 6487-6493.
-

- 31. T.-Y. Hwang, J. Lee, H.-R. Lim, S.J. Jeong, G.-H. An, J. Kim, Y.-H. Choa, Ceram. Int. 43 (2017) 3879-3884.
-

- 32. L. Peng, L.Z. Li, X.X. Zhong, Y.B. Hu, S.M. Chen, J. Magn. Magn. Mater. 428 (2017) 73-77.
-

- 33. Z.Y. Zhang, X.X. Liu, X.J. Wang, Y.P. Wu, R. Li, J. Alloys Compd. 525 (2012) 114-119.
-

- 34. H.M. Khan, M.U. Islam, Y.B. Xu, M. Asif Iqbal, I. Ali, M. Ishaque, M.A. Khan, J. Sol-Gel. Sci. Technol. 75 (2015) 305-312.
-

- 35. Z. Lalegani, A. Nemati, J. Mater. Sci.: Mater. Electron. 26 (2015) 2134-2144.
-

- 36. C.C. Liu, X.C. Kan, F. Hu, X.S. Liu, S.J. Feng, J.Y. Hu, W. Wang, K.M. Ur Rehman, M. Shezad, C. Zhang, H.H. Li, S.Q. Zhou, Q.Y. Wu, J. Alloy. Compd. 785 (2019) 452-459.
-

- 37. Y.J. Yang, D.H. Huang, J.X. Shao, F.H. Wang, Chin. J. Phys. 57 (2019) 250-260.
-

- 38. A. Majeed, M.A. Khan, F. ur Raheem, A. Hussain, F. Iqbal, G. Murtaza, M.N. Akhtar, I. Shakir, M.F. Warsi, J. Magn. Magn. Mater. 408 (2016) 147-154.
-

- 39. V.N. Dhage, M.L. Mane, M.K. Babrekar, C.M. Kale, K.M. Jadhav, J. Alloys Compd. 509 (2011) 4394-4398.
-

- 40. Y.J. Yang, X.S. Liu, D.L. Jin, Y.Q. Ma, Mater. Res. Bull. 59 (2014) 37-41.
-

- 41. C. Herme, S.E. Jacobo, P.G. Bercoff, B. Arcondo, Hyperfine Interact. 195 (2010) 205-212.
-

- 42. S.W. Lee, S.Y. An, In-Bo Shim, C.S. Kim, J. Magn. Magn. Mater. 290-291 (2005) 231-233.
-

- 43. Z. Yang, C.S. Wang, X.H. Li, H.X. Zeng, Mater. Sci. Eng. B 90 (2002) 142-145.
-

- 44. B.K. Rai, S.R. Mishra, V.V. Nguyen, J.P. Liu, J. Alloys Compd. 550 (2013) 198-203.
-

- 45. TDK Electronics, in “TDK Products Catalog: Ferrite Magnets” (TDK Electronics, 2011) p.3.
- 46. A. Goldman, in “Modern Ferrite Technology” (Springer, 2006) p.235.
- 47. Z.G. Zhou, in “Ferrite Magnetic Materials” (Science Press, 1981) p.646.
- 48. W.Y. Zhou, in “Design Technology of Permanent Magnet Ferrite and Magnetic Fluid” (University of Electronic Science and Technology Press, 1991) p.34.
- 49. TDK Electronics, in “TDK Products Catalog: Ferrite Magnets” (TDK Electronics, 2014) p.4.
- 50. F. Aen, M.F. Wasiq, M.U. Rana, H.M. Khan, H.A. Khan, Ceram. Int. 42 (2016) 16077-16083.
-

- 51. A.M. Van Diepen, F.K. Lotgering, J. Phys. Chem. Solids 35 (1974) 1641-1643.
-

 This Article
This Article
-
2020; 21(4): 416-424
Published on Aug 30, 2020
- 10.36410/jcpr.2020.21.4.416
- Received on Dec 28, 2019
- Revised on Apr 21, 2020
- Accepted on May 4, 2020
 Services
Services
- Abstract
introduction
experimental details
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Yujie Yang
-
Engineering Technology Research Center of Magnetic Materials, School of Physics & Materials Science, Anhui University, Hefei 230601, P. R. China
Tel : +86 551 63861257
Fax: +86 831 63861257 - E-mail: loyalty-yyj@163.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.