- Impacts of praseodymium substitution on structural, spectral, magnetic and electrical properties of strontium W-type hexaferrites
Yujie Yang*, Xiansong Liu, Shuangjiu Feng, Qingrong Lv, Xucai Kan and Ruiwei Zhu
Engineering Technology Research Center of Magnetic Materials, School of Physics & Materials Science, Anhui University, Hefei 230601, P. R. China
Praseodymium substituted strontium W-type
hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) were prepared via the conventional ceramic technique.
XRD analysis of W-type hexaferrites with Pr content (x) of 0.00 ≤ x ≤ 0.24
shows the single W-type hexaferrite phase. However, for the W-type hexaferrites
with Pr content (x) ≥ 0.32, the impurity phase (α-Fe2O3)
is detected. FE-SEM images show that the grains are platelet-like shapes. The
saturation magnetization (Ms), remanent magnetization (Mr)
and magneton number (nB) first increase with Pr content (x) from
0.00 to 0.16, and then decrease when Pr content (x) ≥ 0.16. The Mr/Ms
ratio, magnetic anisotropy field (Ha), coercivity (Hc)
and maximum energy product [(BH)max] increase with Pr content
(x) from 0.00 to 0.40. While the first anisotropy constant (K1)
increases with Pr content (x) from 0.00 to 0.16, and then decreases when Pr
content (x) ≥ 0.16. The DC electrical resistivity (ρ) decreases with Pr
content (x) from 0.00 to 0.40.
Keywords: W-type hexaferrites, Conventional ceramic technique, X-ray diffraction, Magnetic properties, Electrical resistivity
Hexagonal ferrites are playing a major role in the field
of permanent magnetic materials in the market because of
their low costs, high saturation magnetization, high
coercivity, and perfect chemical stability [1, 2]. Hexagonal ferrites have been
extensively used in microwave devices and electromagnetic wave absorbers
due to their high saturation magnetization, tunable dielectric properties, high
planar magnetic anisotropy, and perfect chemical stability [3]. Hexagonal
ferrites are classified into six different possible types based on the chemical
and crystalline structure, which include M, W, Y, X, Z
and U hexaferrites, depending upon their crystal
structure [4]. The strontium W-type hexaferrites (SrMe2Fe16O27)
have a crystalline structure as stacking of R (composition: SrFe6O11)
and S (spinel block, with composition: Fe6O8) stocks. The
main structure of W-type hexaferrites consists of SSRS*S*R*. The asterisk means
that the corresponding block is rotated 180o alone the c-axis [5].
In the W-type hexaferrites, the Fe3+ ions are distributed among
seven different crystal positions, such as four octahedral positions (12k, 4fVI,
6g, and 4f), two tetrahedral positions (4e, 4fIV), and one
hexahedral position (2d) [6].
Various methods have been proposed to synthesize the
W-type hexaferrites, such as the chemical co-preci- pitation method [7], sol-gel technique
[8], sol-gel auto combustion technique [9], citrate method [10], conven- tional ceramic technique [11], and
glass crystallization method [12]. In the present work,
the conventional ceramic technique has been used to synthesize the W-type
hexaferrites due to its numerous virtues, namely, simplicity, high productive
and well controllable grain size as compared with other methods.
Rare earth elements (RE) have typical relaxation
characteristics, which may affect the electromagnetic properties of
the ferrites [13]. Ahmad et al. studied La substituted W-type hexaferrites
BaZn2LaxFe16-xO27 (0 ≤ x ≤
1.0) synthesized by co-precipitation method and observed that the saturation
magnetization and remanence decreased with La content (x) from 0 to 1.0, while
the coercivity (Hc) increased with La content (x) from 0 to 1.0 [14]. Sadiq et al. worked on
Ce-substituted W-type hexagonal ferrites Sr3-xCexFe16O27
(0 ≤ x ≤ 0.10) prepared
by sol-gel method and found that with the doping of Ce3+ ions, the
grain size decreased and the saturation magnetization and coercivity increased
[15]. Xu et al. synthesized Nd3+ doped
W-type ferrites Ba1-xNdxCo2Fe16-xO27
(0.00 ≤ x ≤ 0.25) by sol-gel method,
and observed that the real part of complex permeability (ɛ′) and imaginary part
(ɛ″) increased with the addition of Nd3+ amount, while the imaginary
part of complex permittivity (μ″) increased and real part (μ′) went down when
Nd3+ ions doped Ba2+ ions [16]. Wang et al. fabricated
Sm-substituted W-type barium hexaferrites Ba1-xSmxCo2Fe16O27
(0.0 ≤ x ≤ 0.2) via conventional solid-state reaction, and found that ɛ′ and ɛ″
increased slightly with Sm3+ ions doping and the values of μ′ and μ″ were improved significantly when x = 0.15 [17].
Aen et al. synthesized the
Ho-substituted W-type hexagonal ferrites Ba1-xHoxCo2Fe16O27
(0.0 ≤ x ≤ 0.1) using the sol-gel auto combustion technique, and observed that
the DC electrical resistivity increased with increasing Ho3+ content
(x) and the Ho3+ substitution caused the dielectric constant (real
and imaginary part of complex permeability) and loss tangent to decrease [18].
Huang et al. have prepared Er3+-substituted W-type barium ferrites
Ba1-xErx(Zn0.3Co0.7)2Fe16O27
(0.00 ≤ x ≤ 0.20) by polymer absorbent combustion and observed that all XRD
patterns showed the pure phase of W-type barium ferrite when x ≤ 0.15, while
the impurity phase of ErFeO3
appeared when x = 0.20; and the electromagnetic properties were significantly improved when x =
0.10 [19]. Khan et al. synthesized Ce-Mn substituted W-type hexagferrites Sr1-xCexCo2MnyFe16-yO27
(0.00 ≤ x ≤ 0.06, 0.0 ≤ y ≤ 0.6) by chemical co-precipitation method and found
that the saturation magnetization, remanence, squareness ratio and coercivity
increased with increasing Ce-Mn concentration up to a certain substitution level, while the room temperature
resistivity decreased with increasing
Ce-Mn concentration [20]. Khan et al. prepared the Nd-Ni substituted
W-type hexagferrites Sr1-xNdxCo2NiyFe16-yO27
(0.00 ≤ x ≤ 0.10, 0.0 ≤ y ≤ 1.0) via the chemical co-precipitation method and observed that the saturation
magnetization, remanence, squareness
ratio and coercivity increased with increasing Nd-Ni concentration up to a x =
0.025, y = 0.25 and then decreased continuously, the increase in magnetic
properties is helpful for their applications in magnetic recording media [21].
However, the impact of praseodymium substitution on
magnetic properties of strontium W-type hexaferrites has not been
reported. Thus, in this work, we have fabricated praseodymium substituted
strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) by conventional
ceramic technique. Effects of praseodymium substitution
on the microstructural, spectral, magnetic and
electrical properties of strontium W-type hexaferrites were
systematically investigated for the first time.
Strontium carbonate (SrCO3) (99.5%),
praseodymium oxide (Pr6O11) (99.9%), zinc
oxide (ZnO) (99%), cobalt oxide (CoO) (99%), iron oxide (Fe2O3)
(99.3%) were used as raw materials. All regents were used as
received, i.e. no further purification of the chemicals was
carried out. The praseodymium substituted strontium W-type
hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) were synthesized by the conventional
ceramic technique. The raw materials were stoichimetrically weighed,
and then ball-milled in water for 10 h in a ball mill. The ball-milled powder
was dried, and pressed into pellets with a diameter of 30 mm and a thickness of
16 mm. The pellets were calcined in a muffle furnace at 1,280 oC for
3.0 h in air to obtain W-type hexaferrite phase. Subsequently, the calcined
pellets were crushed by a vibration mill to obtain fine powders through a sieve
with a 200 mesh. And then, the crushed magnetic powders were
pressed into pellets with a diameter of 20 mm and a thickness of 8 mm. The
pellets were finally sintered in a muffle furnace at 1,205 oC
for 2.0 h in air, and used for the DC electrical resitivity measurement.
The phase and crystal structure of strontium W-type hexaferrites
were determined from the X-ray diffraction (XRD)
patterns. The X-ray diffraction patterns were recorded from a Rigaku X-ray
diffractomer equipped with Cu Kα (λ = 1.5406 Å) radiation. The 2θ
angles were scanned over a range between 20o and 80o with
equal steps of 0.01o. Fourier transform infrared (FTIR, Nicolet
6700, Thermo Scientific) was performed to investigate the metal ion stretching
and absorption bands in the wave number range of 400 to 4,000 cm-1. Field
emission scanning electron microscopy (FE-SEM, HITACHI
S-4800) was employed to determine the grain size and
morphology of strontium W-type hexaferrites. Magnetic
properties were measured at room temperature using
vibrating sample magnetometer (VSM) at the maximum magnetic field of 18800 Oe.
DC electrical resitivity (ρ) was measured at room temperature by two probe
method (Resistivity testing system, Ningbo rooko FT-353).
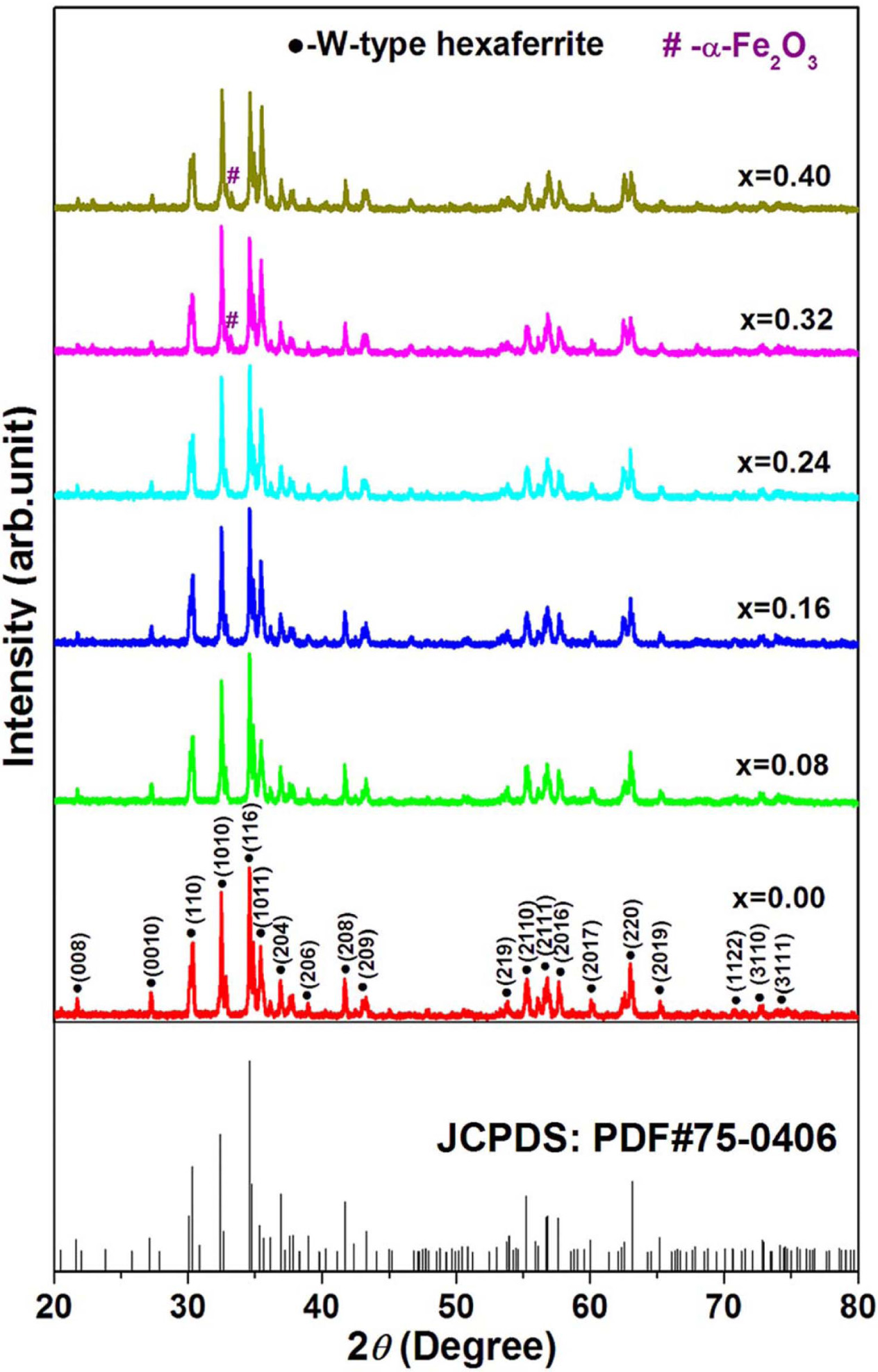
Fig. 1 presents the XRD patterns for strontium W-type
hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40). XRD patterns of all samples were indexed using the
standard pattern for W-type hexagonal ferrite (JCPDS card no.
75-0406). It can be observed from Fig. 1 that the W-type
hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
with Pr content (x) ≤ 0.24 are single-phased W-type hexaferrites, while for the
W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
at Pr content (x) ≥ 0.32, the W-type hexaferrite phase is a major phase and the
secondary phase α-Fe2O3 (JCPDS card no. 87-1166) is
observed.
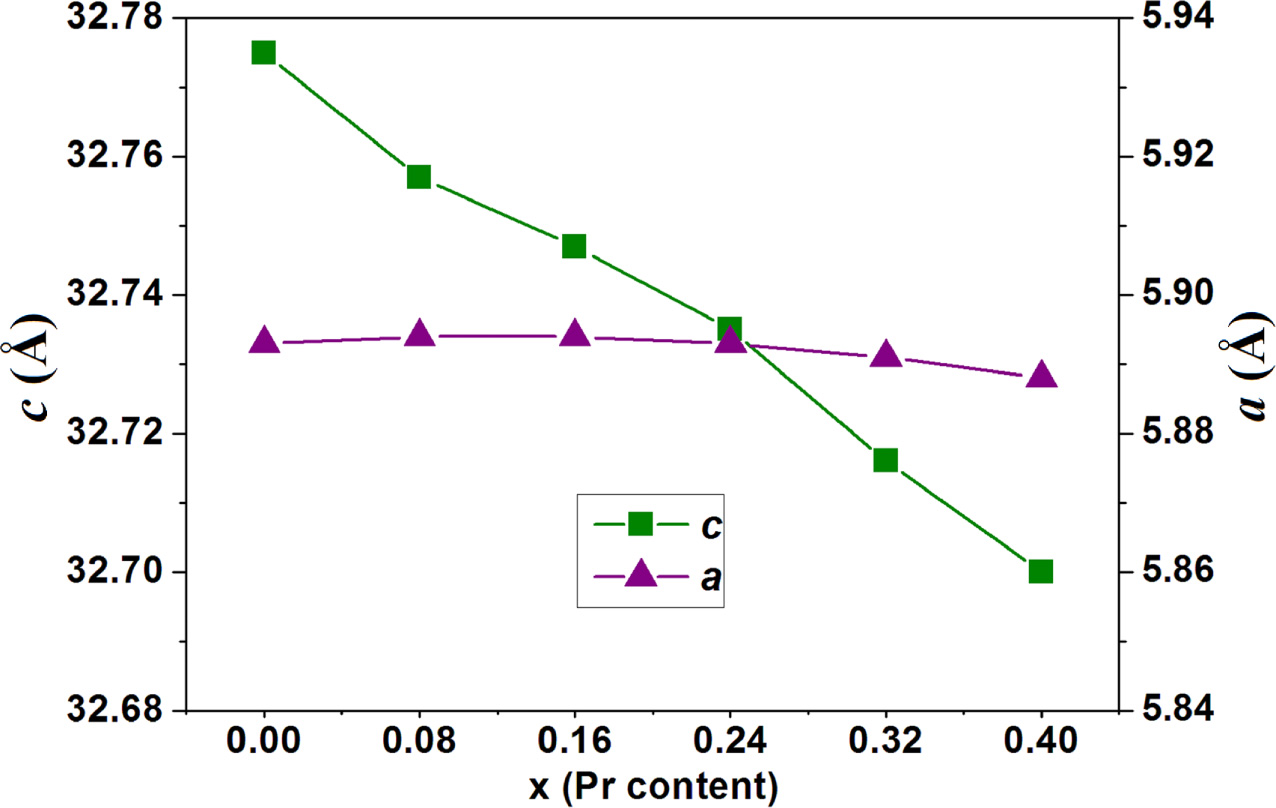
For W-type hexaferrites, lattice parameters a and c
were obtained from the values of dhkl corresponding to (1010)
and (116) planes using the following equation [22]:
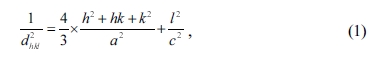
where dhkl is the
inter planner spacing in the XRD pattern, and h, k and l are
the Miller indices. The variations of lattice parameters (a and c)
for the strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) are depicted in Fig. 2. By substituting the Sr2+ ions
with Pr3+ ions, the lattice parameter a reflects less
variation whereas the lattice parameter c decreases with increasing Pr
content (x) from 0.00 to 0.40. This is in agreement with the fact
that the hexaferrites display constant lattice parameter a and
changeable lattice parameter c [23]. For the strontium W-type
hexaferrites with Pr substitution, the decrease in lattice parameter c
is due to the difference in the ionic radii (Δr) of metal ions and the
number of ionic substitutions. It is known that the ionic radii of Sr2+,
Pr3+, Fe3+ and Fe2+ are 1.180 Å, 0.990 Å,
0.645 Å, and 0.78 Å, respectively. Substitution of Sr2+ (r =
1.180 Å) by Pr3+ (r = 0.990 Å) makes a negative difference in
the ionic radii of Δr = -0.190 Å. For Pr substituted W-type hexaferrites
Sr1-xPrxZn0.8Co1.2Fe16O27,
in order to compensate the excessive positive charges because of the
substitution of Sr2+ by Pr3+, some Fe3+ ions (r
= 0.645 Å) convert into Fe2+ ions (r
= 0.780 Å). This makes a positive
difference in the ionic radii of Δr = +0.135 Å. The above
two results exhibit that the crystal structures of strontium W-type hexaferrites
are contracted after being substituted by Pr3+ ions and the lattice
parameter c decreases with increasing Pr content (x).
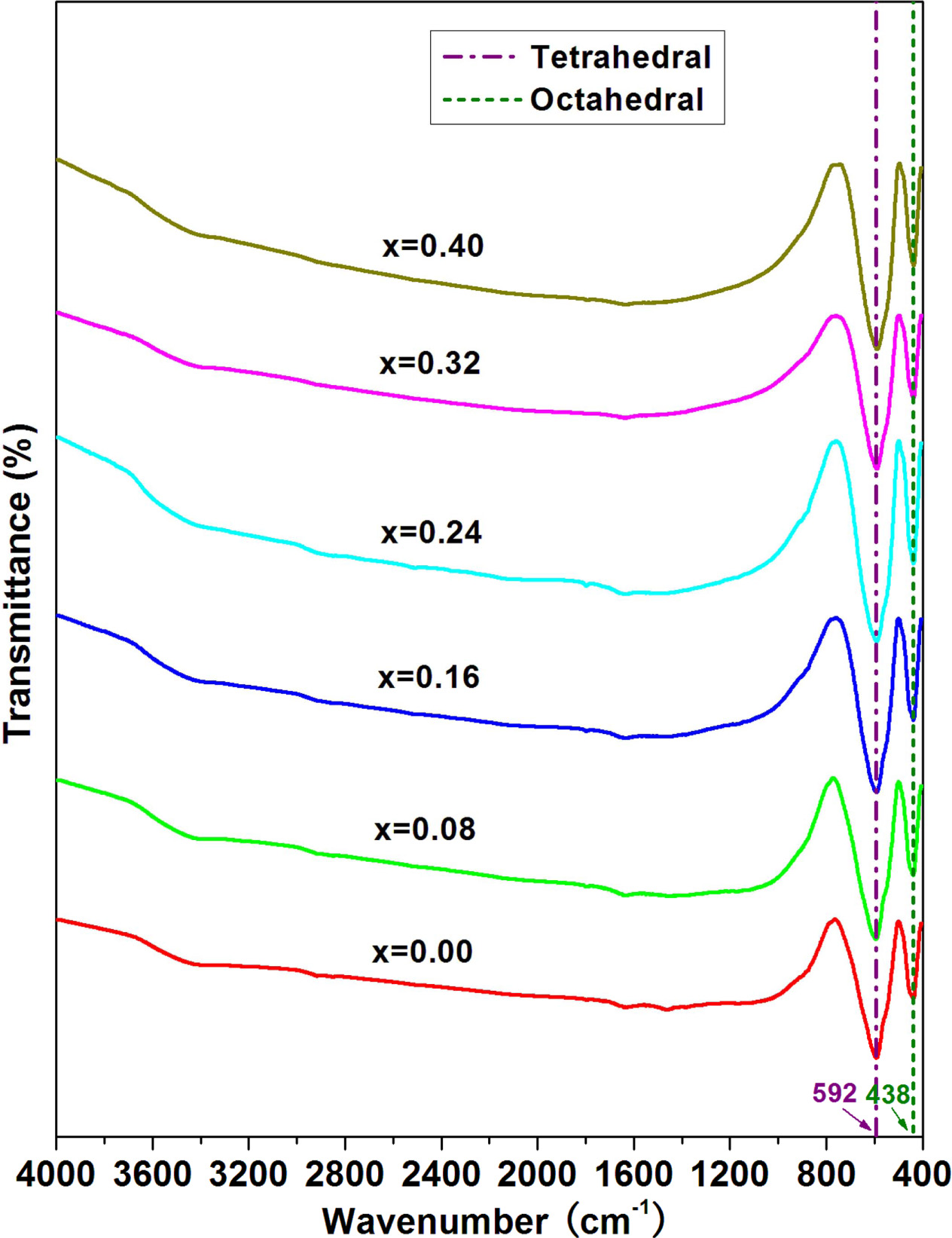
The FT-IR spectra of strontium W-type hexaferrites were
recorded in the wavenumber range of 400-4000 cm-1.
FT-IR spectra for strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) are illustrated in Fig. 3.
The absorption bands in the frequency range of 400-800 cm-1 are due to vibration
bonds of the hexagonal ferrites [24]. The
absorption band in the range 590-594
cm-1 is
caused by the stretching vibrations of tetrahedral metal ion and oxygen
bonding. And The absorption band in the range 438-440 cm-1 is
attributed to the stretching vibrations of octahedral metal ion and oxygen
bonding [25]. As seen from Fig. 3, the positions of
absorption bands do not change obviously, and the normal vibration mode of
tetrahedral cluster is higher than that of octahedral cluster. This is assigned
to shorter bond length of tetrahedral cluster and longer bond length of
octahedral cluster [26]. For all strontium W-type hexaferrites with different
Pr content (x), the absorption band at about 1,629 cm-1 and
about 3,389 cm-1 are
due to the stretching vibration of surface hedroxy group (-OH) because of water
in the W-type hexaferrites acquired from the process of preparation [27, 28].
Fig. 4 provides the FE-SEM micrographs of strontium
W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
(x=0.00, 0.16, and 0.32) under 9k magnification. It can be noted that Pr
content (x) has no significant effect on the grain shape and morphology. The
grains are platelet-like morphology with the average grain
size of about 2.6 μm.
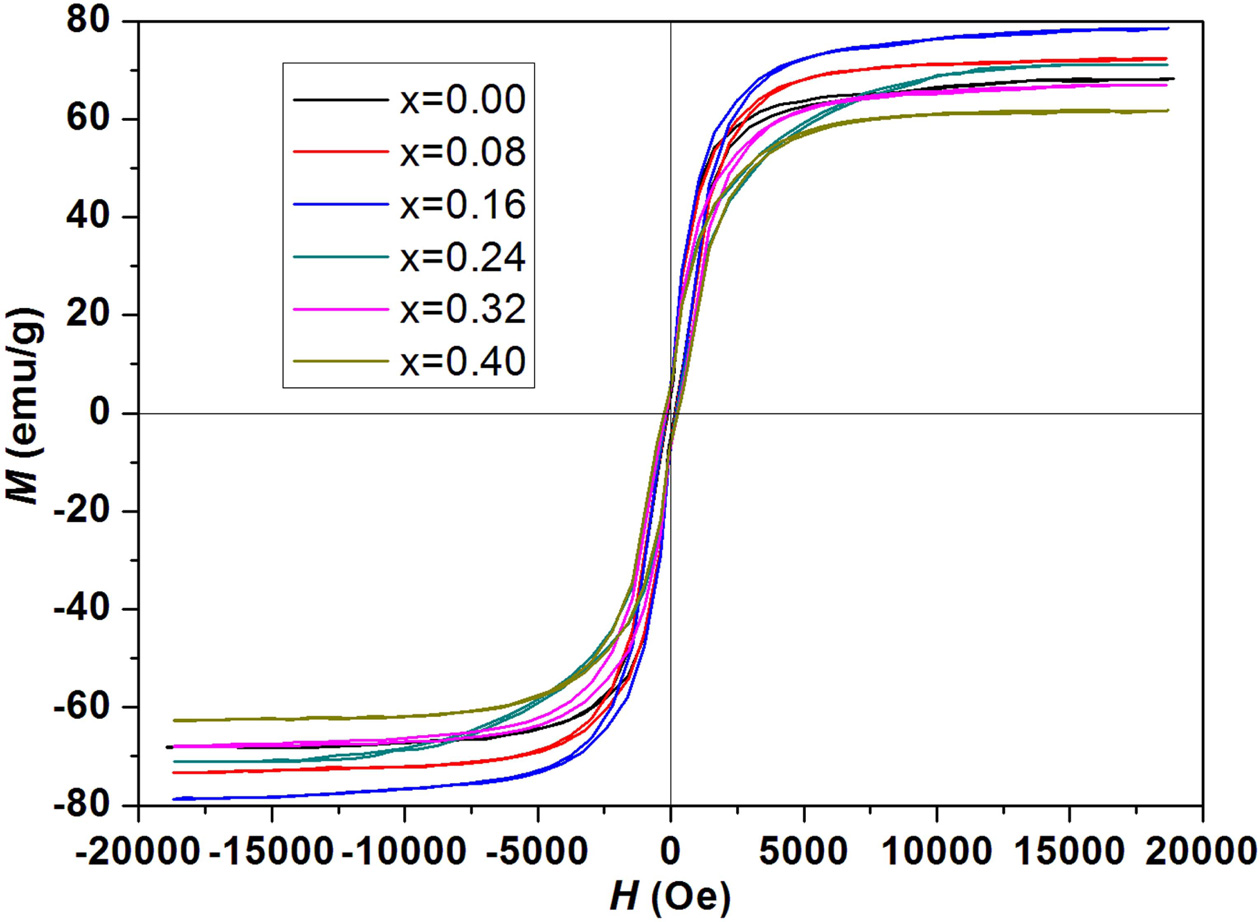
Magnetic hysteresis loops of strontium W-type hexaferrites
Sr1-xPrxZn0.8Co1.2Fe16O27
(0.00 ≤ x ≤ 0.40) are represented in Fig. 5. The values of hysteresis
parameteres are calculated from magnetic hysteresis loops with different Pr
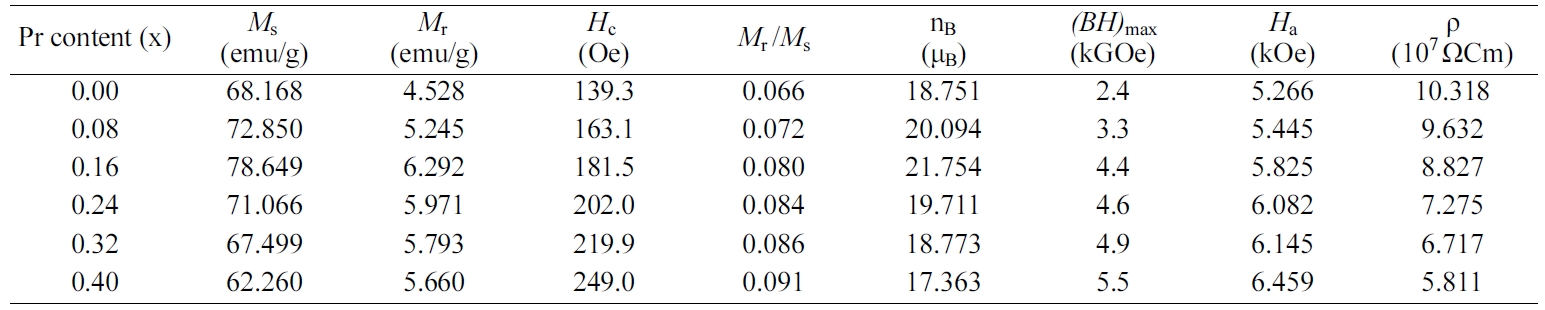
content (x) and are tabulated in Table 1.
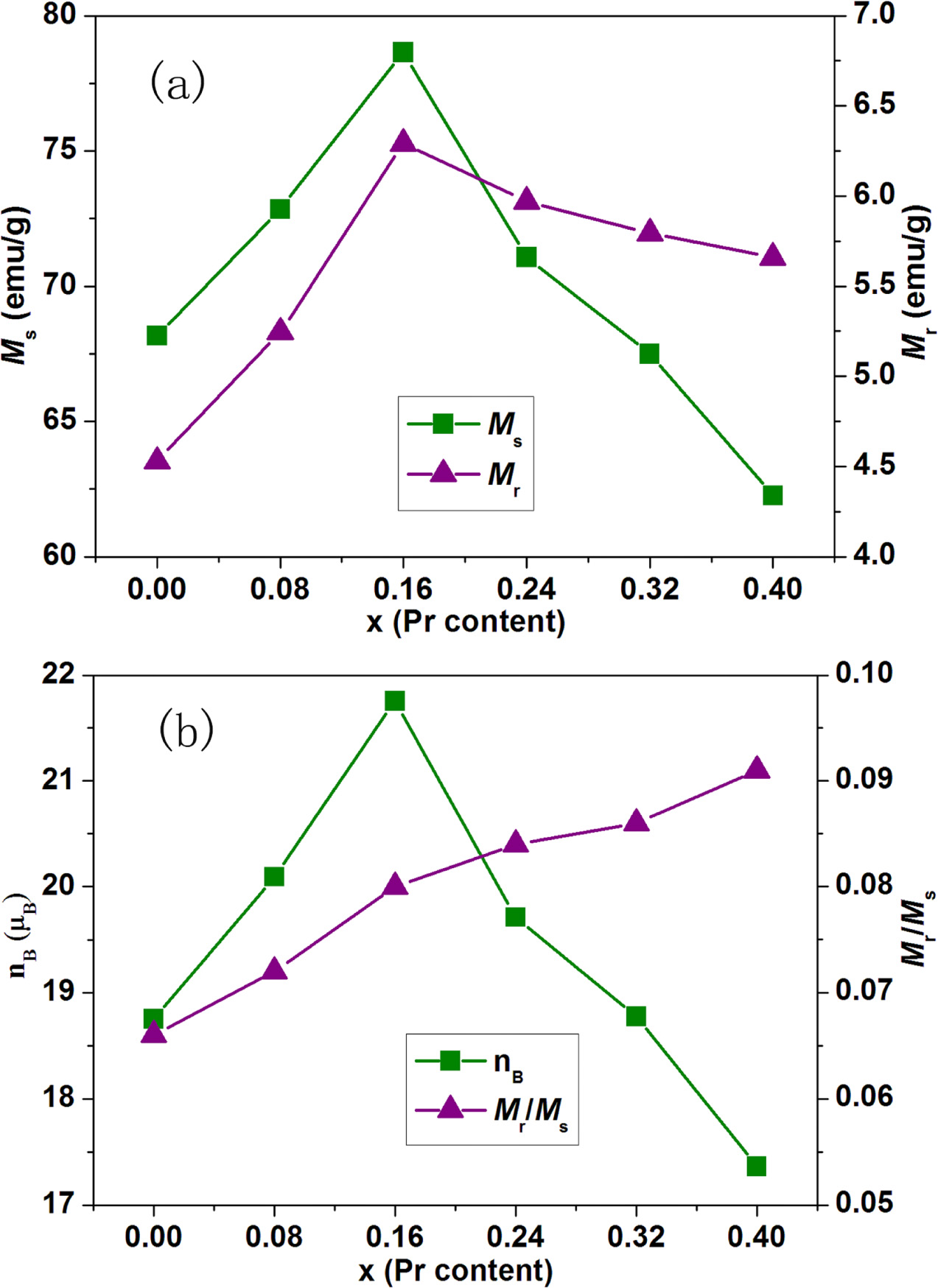
Fig. 6(a) describes the variations of saturation magnetization
(Ms) and remanent magnetization (Mr) as a
function of Pr content (x) for strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27.
It is observed from Fig. 6(a) that the values of Ms and Mr
first increase from 68.168 and 4.528 emu/g at x = 0.00 to 78.649
and 6.292 emu/g at x = 0.16, respectively; and then decrease with Pr content
(x) from 0.16 to 0.40. The magnetic moment of Fe3+ and Fe2+ ions
is 5.0 μB and 4.0 μB, respectively. On the one hand, the
increase in Ms and Mr with Pr content (x)
from 0.00 to 0.16 can be attributed to the following reason. When Pr3+
ions substitute the Sr2+ ions in W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27,
the shrinkage of lattice parameter c as shown in Fig. 2 results in the
decrease of Fe-O which enhances the super-exchange interactions among the
sublattices [16, 29]. Therefore, Ms and Mr are
increased for the Pr substituted W-type hexaferrites (0.00 ≤ x ≤ 0.16). On the
other hand, with increasing Pr content (x) from 0.16 to 0.40, the
decreasing in Ms and Mr can be assignable
to the below three factors. Firstly, for the hexaferrites, the Fe3+
ions are arranged collinearly due to the superexchange
interactions. For the Pr substituted W-type
hexaferrites, in order to compensate the excessive positive
charges, the substitution of Sr2+ by Pr3+ causes some Fe3+
ions (5.0 μB) to convert into Fe2+ ions (4.0 μB).
Abundance of Fe2+ ions will cause the collinearity of the
superexchange interactions to break with increasing Pr content (x) from 0.16 to
0.40. This is spin canting or non-collinear magnetic order which results in the
decrease of Ms and Mr [33, 34]. Secondly,
the valence change of Fe3+ ions (5.0 μB) into Fe2+ ions
(4.0 μB) in order to balance the excessive positive charges
because of Sr2+ ions substituted by Pr3+ ions leads to
the decrease of net molar magnetic moment. This is magnetic dilution [29, 30].
As a result, the values of Ms and Mr decrease
continuously. Thirdly, for the W-type hexaferrites with Pr content (x) from
0.32 to 0.40, the secondary phase α-Fe2O3 is present as
shown in Fig. 1. As a secondary phase in the W-type hexaferrites, α-Fe2O3
has no contribution to the increase of Ms and Mr,
and can cause the values of magnetization to dilute. This leads to the decrease
of Ms and Mr.
The Bohr magneton number (nB) of strontium
W-type hexaferrites with different Pr content (x) was calculated by the
following relation [31]:

where M.W. is the molecular
weight and Ms is the saturation magnetization. The Mr/Ms
ratio is calculated from magnetic data. The variations of
magneton number (nB) and Mr/Ms
as a function of Pr content (x) for strontium
W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
are mentioned in Fig. 6(b). It is observed that the value of nB
increases from 18.751 μB at x = 0.00 to 21.754 μB at x =
0.16, and then decreases with increasing Pr content (x) from 0.16 to 0.40. nB
has the same varying trend as Ms exhibited in Fig. 6(a). This
shows that magnetic moment is the main mechanism behind the change of Ms.
The Mr/Ms ratio is known as squareness
ratio. As seen from Fig. 6(b), the value of Mr/Ms
ratio increases from 0.066 at x
= 0.00 to 0.091 at x = 0.40. This shows that the strontium W-type hexaferrites with
different Pr content (x) are
multi-domain structure.
The magnetic anisotropy field (Ha) and
magneto- crystalline anisotropy
constant (K1) are determined according to the law of approach
to saturation [32]. The relationship between the magnetization (M) and
sufficiently high magnetic fields (H) is expressed as follows [33]:
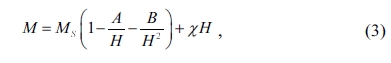
where Ms is
saturation magnetization, A is a constant arising from the
inhomogeneities, the constant B is related with
the magnetic crystalline anisotropy constant, H is
the magnetic field in this region, and χ represents the high-field
differential susceptibility. The constant A is approximate to zero. χ
is neglected at high magnetic field. The M vs. 1/H2 plot
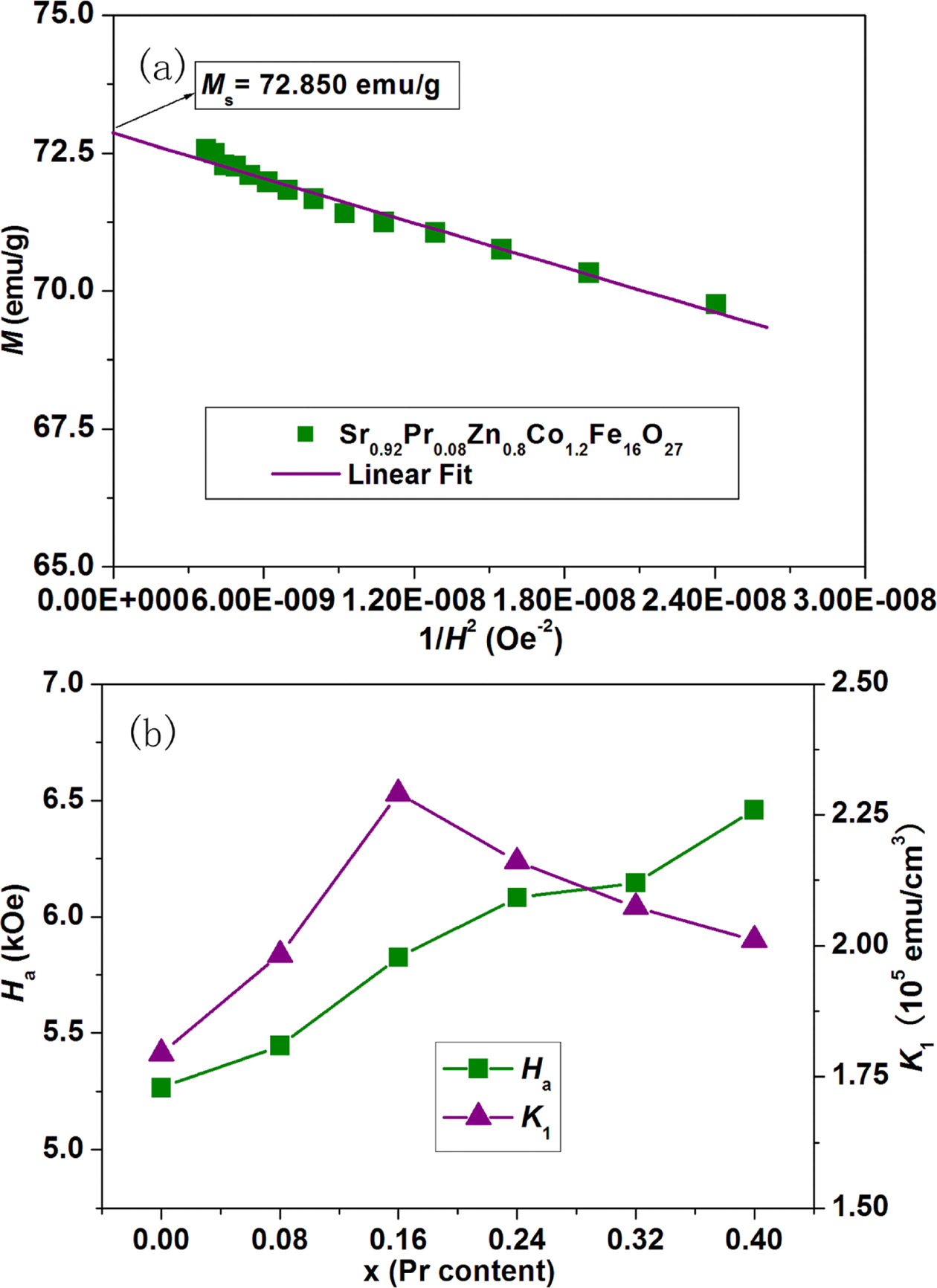
in the high magnetic field gives a straight line. Fig. 7(a) reveals the plot of magnetization of strontium W-type
hexaferrite Sr0.92Pr0.08Zn0.8Co1.2Fe16O27
as a function of 1/H2. The slope
gives the value of B. The first anisotropy constant (K1)
can be calculated using the following equation [34]:
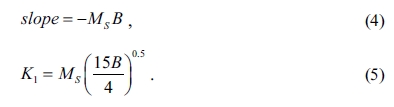
The calculated values of K1 can be used
to estimate the magnetic anisotropy field (Ha) of the W-type
hexaferrites by using the below expression [34]:

The variations of Ha and K1
as a function of Pr content (x) for strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
are exhibited in Fig. 7(b). It could be seen that the value of K1
initially increases from 1.795×105 emu/cm3 at x = 0.00 to
2.291×105 emu/cm3 at x = 0.16, and then decreases when Pr
content (x) ≥ 0.16; while the value of Ha increases from
5.266 kOe at x = 0.00 to 6.459 kOe at x = 0.40 as a result of increasing Pr
content (x). The increase of Ha can ascribed to the following
two reasons. Firstly, for the hexaferrites, the low symmetry of trigonal
bipyramidal 2b site is the main contributor to the stronger uniaxial
magnetocrystalline anisotropy [35]. Substitution of Sr2+ (r =
1.180 Å) by Pr3+ (r = 0.990 Å) can lead to greater lattice
distortion and lower symmetry of trigonal bipyramidal 2b site [36]. This causes
Ha to increase. Secondly, it has been reported that Fe2+
ions could increase the magnetic anisotropy field because of strong
magnetocrystalline anisotropy of Fe2+ ions [37]. The number of Fe2+
ions increases with increasing Pr content (x) because the substitution of
Sr2+ by Pr3+ causes some Fe3+ ions to convert
into Fe2+ ions in order to compensate the excessive positive
charges. Thus, Ha is enhanced.
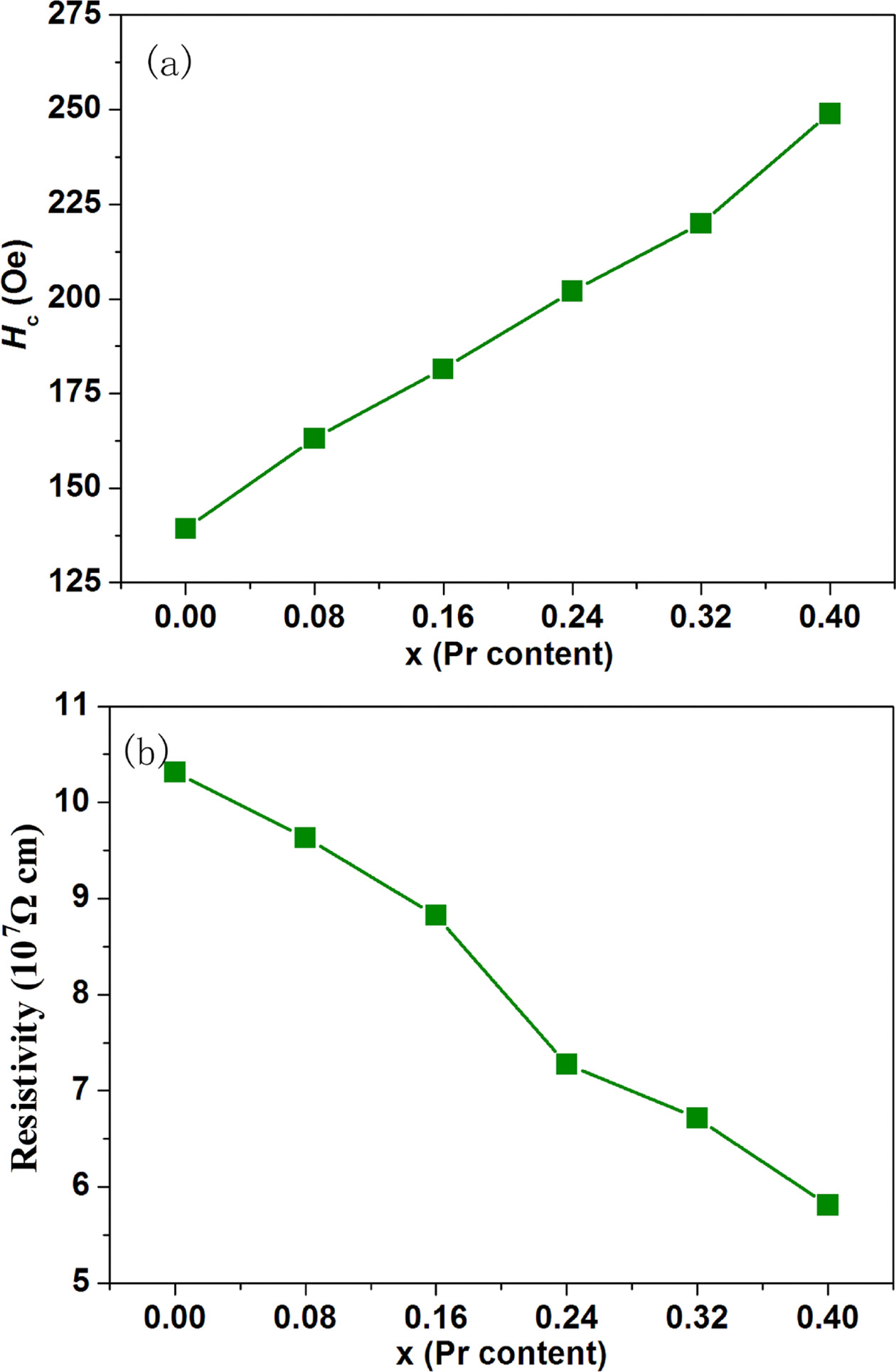
Fig. 8(a) represents the variation of coercivity (Hc)
as a function of Pr content (x) for strontium W-type hexaferrites Sr1-xPrx
Zn0.8Co1.2Fe16O27. It is worthy to
note that with increasing Pr content (x), the value of Hc
enhances from 139.3 Oe at x = 0.00 to 249.0 Oe at x = 0.40. The coercivity (Hc)
is corrected with the intrinsic magnetic parameters and microstructure based on
the following relation [38]:

where α is a microstructure factor
that raises with decreasing grain size, N is called the
demagnetizing factor determined by many factors one of which is aspect ratio
and μ0 is the permeability of free space. Fig. 4 shows
that the platelet-like shapes and average grain size are basically unchanged
with Pr substitution. Hence, α and N basically remain
constant. According to the relation (7), we can conclude
that the enhancement of coercivity (Hc) is primarily
due to the increase of magnetic anisotropy field (Ha) as
displayed in Fig. 7(b). As seen from Table 1, the value of
maximum energy product [(BH)max]
for strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
increases with increasing Pr content (x) from 0.00 to 0.40. It is well known
that the maximum energy product can be
obtained by multiplying the corresponding B and H values at the point of
operation on the demagnetizing curves [39]. Therefore, the values of the
remanent magnetization (Mr) and coercivity (Hc)
have effect on the value of (BH)max.
As seen from Fig. 6(a) and Fig. 8(a), the changing trend of (BH)max are owing to
both the variation of Mr and variation of Hc.
Therefore, magnetic properties of the strontium W-type hexaferrites can be
increased with the substitution of Pr3+ ions for Sr2+
ions.
The variation of DC electrical resitivity (ρ) as a
function of
Pr content (x) for strontium W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27
is revealed in Fig. 8(b) and the values of ρ are also listed in Table 1.
It can be observed that ρ is obviously affected by Pr
content (x). With the increase of Pr content (x), the electrical resitivity (ρ)
decreases from 10.318×107 Ωcm at x = 0.00 to
5.8113×107 Ωcm at x =0.40. It is reported that the conductivity in
the hexaferrites can be attributed to the
hopping between Fe3+ and Fe2+ ions at the octahedral sites
[39]. For Pr substituted W-type hexaferrites Sr1-xPrxZn0.8Co1.2Fe16O27,
in order to compensate the excessive positive charges because of the
substitution of Sr2+ by Pr3+, some Fe3+ ions
convert into Fe2+ ions. This increases the number of Fe2+ ions
which leads to the increase of the hopping probability between the Fe3+
and Fe2+ ions. Thus, the above factors cause the electrical
resitivity (ρ) to decrease.
|
Fig. 1 XRD patterns of strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40). |
|
Fig. 2 Variations of lattice parameters (c and a) for strontium Wtype hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40). |
|
Fig. 3 FT-IR spectra for strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40). |

|
Fig. 4 FE-SEM micrographs of strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 with Pr content (x) of (a) x=0.00, (b) x=0.16, and (c) x=0.32. |
|
Fig. 5 Magnetic hysteresis loops of strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40). |
|
Fig. 6 Variations of (a) saturation magnetization (Ms) and remanent magnetization (Mr), and (b) magneton number (nB) and Mr/Ms as a function of Pr content (x) for strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27. |
|
Fig. 7 (a) A plot of magnetization of strontium W-type hexaferrite Sr0.92Pr0.08Zn0.8Co1.2Fe16O27 as a function of 1/H2, and (b) variations of the magnetic anisotropy field (Ha) and first anisotropy constant (K1) as a function of Pr content (x) for strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27. |
|
Fig. 8 Variation of (a) coercivity (Hc), and (b) DC electrical resitivity (ρ) as a function of Pr content (x) for strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27. |
|
Table 1 Values of the hysteresis parameteres and DC electrical resitivity (ρ) for strontium W-type hexaferrites Sr1-x PrxZn0.8Co1.2Fe16O27 (0.00 ≤ x ≤ 0.40). |
This work was supported by the National Natural Science Foundation of
China (Nos. 51872004, 51802002),
Education Department of Anhui Province (Nos. KJ2013B293, KJ2018A0039).
- 1. L. Lechevallier, J.M. Le Breton, J.F. Wang, and I.R. Harris, J. Magn. Magn. Mater. 269[2] (2004) 192-196.
-

- 2. P. Shepherd, K.K. Mallick, and R.J. Green, J. Magn. Magn. Mater. 311[2] (2007) 683-692.
-

- 3. S.Wei, Y. Liu, H. Tian, H. Tong, Y.Liu, and B.Xu, J. Magn. Magn. Mater. 377 (2015)[1] 419-423.
-

- 4. R.C. Pullar, Prog. Mater. Sci. 57[7] (2012) 1191-1334.
-

- 5. D.M. Hemeda, A. Al-Sharif, and O.M. Hemeda, J. Magn. Magn. Mater. 315[1] (2007) L1-L7
-

- 6. X.Z. Zhou, I. Horio, A.H. Morrish, Z.W. Li, and K. Hanava, IEEE Trans. Magn. 27[6] (1991) 4651-4653.
-

- 7. M.J. Iqbal, R.A. Khan, S. Mizukami, and T. Miyazaki, J. Magn. Magn. Mater. 323[16] (2011) 2137-2144.
-

- 8. L. Deng, L. ding, K. Zhou, S. Huang, Z. Hu, and B.C. Yang, J. Magn. Magn. Mater. 323[14] (2011) 1895-1898.
-

- 9. M. Ahmad, I. Ali, F. Aen, M.U. Islam, M. N. Ashiq, S. Atiq, W. Ahmad, and M.U. Rana, Ceram. Int. 38[2] (2012) 1267-1273.
-

- 10. S. Ruan, B. Xu, H. Suo, F. Wu, S. Xiang, and M. Zhao, J. Magn. Magn. Mater. 212[1-2] (2000) 175-177.
-

- 11. F. Lv, X. Liu, S. Feng, K. Huang, X. Niu, X. Huang, F. Huang, Y. Ma, S. Jiang, and Y. Wu, Mater. Lett. 157[20] (2015) 277-280.
-

- 12. C. Sürig, K.A. Hempel, R. Müller, and P. Görnert, J. Magn. Magn. Mater. 150[2] (1995) 270-276.
-

- 13. F. Gu, G. Ji, J. Xu, H. Zou, S. Gan, and X. Xu, J. Magn. Magn. Mater. 324[6] (2012) 1209-1213.
-

- 14. M. Ahmad, F. Aen, M.U. Islam, S.B. Niazi, and M.U. Rana, Ceram. Int. 37[8] (2011) 3691-3696.
-

- 15. I. Sadiq, I. Khan, F. Aen, M.U. Islam, and M.U. Rana, Physica B 407[8] (2012) 1256-1261.
-

- 16. J. Xu, H. Zou, H. Li, G. Li, S. Gan, and G. Hong, J. Alloys Compd. 490[1-2] (2010) 552-556.
-

- 17. L.X. Wang, J. Song, Q.T. Zhang, X.G. Huang, and N.C. Xu, J. Alloys Compd. 481[1-2] (2009) 863-866.
-

- 18. F. Aen, M.F. Wasiq, M.U. Rana, H.M. Khan, and H.A. Khan, Ceram. Int. 42[14] (2016) 16077-16083.
-

- 19. X.G. Huang, J. Zhang, H.Z. Wang, S.T. Yan, L.X. Wang, and Q.T Zhang, J. Rare Earths 28[6] (2010) 940-943.
-

- 20. I. Khan, I. Sadiq, M.N. Ashiq, and M.-U.-D. Rana, J. Alloys Compd. 509[31] (2011) 8042-8046.
-

- 21. I. Khan, I. Sadiq, I. Ali, M.-U.-D. Rana, M. Najam-Ul-Haq, A. Shah, I. Shakir, and M.N. Ashiq, J. Magn. Magn. Mater. 397 (2016)[1] 6-10.
-

- 22. P. Kaur, S.B. Narang, and S. Bahel, Ceram. Int. 42[8] (2016) 9830-983.
-

- 23. M.J. Iqbal and M.N. Ashiq, Chem. Eng. J. 136[2-3] (2008) 383-389.
-

- 24. S. Güner, I.A. Auwal, A. Baykal, and H. Süzeri, J. Magn. Magn. Mater. 416 (2016) 261-268.
-

- 25. A.M. Shaikh, S.A. Jadhav, S.C. Watawe, and B.K. Chougule, Mater. Lett. 44[3-4] (2000) 192-196.
-

- 26. A. Pradeep and G. Chandrasekaran, Mater. Lett. 60[3] (2006) 371-374.
-

- 27. A. Ghasemi, Ceram. Int. 42[3] (2016) 4143-4149.
-

- 28. X. Liu, W. Zhong, S. Yang, Z. Yu, B. Gu, and Y. Du, J. Magn. Magn. Mater. 238[2-3] (2002) 207-214.
-

- 29. X.S. Liu, W. Zhong, S. Yang, Z. Yu, B.X. Gu, and Y.W. Dou, Phys. Stat. Sol. A 193[2] (2002) 314-319.
-

- 30. J. Tang, X.S. Liu, K.M. Ur Rehman, M.L. Li, C. Zhang, X.Y. Meng, H.H. Li, and C.C. Liu, J. Mater. Sci.: Mater. Electron. 28[16] (2017) 12086-12091.
-

- 31. M.G. Han, Y. Ou, W.B. Chen, and L.J. Deng, J. Alloys Compd. 474[1-2] (2009) 185-189.
-

- 32. R.E. El Shater, E.H. El-Ghazzawy, and M.K. El-Nimr, J. Alloys Compd. 739[6] (2018) 327-334.
-

- 33. Y. Yang, F.Wang, J. Shao, D. Huang, H. He, A.V. Trukhanov, and S.V. Trukhanov, J. Alloys Compd. 765[20] (2018) 616-623.
-

- 34. Z. Zhou, Z. Wang, X. Wang, X.Wang, J.Zhang, F. Dou, M. Jin, and J. Xu, J. Alloys Compd. 610[20] (2014) 264-270.
-

- 35. I. Bsoul, S.H. Mahood, A.-F. Lehlooh, and A. Al-Jamel, J. Alloys Compd. 551[4] (2013) 490-495.
-

- 36. S. Qunnunkad, Solid State Commun. 138[9] (2006) 472-475.
-

- 37. L. Peng, L.Li, X. Zhong, Y. Hu, and S. Chen, J. Magn. Magn. Mater. 428[8] (2017) 73-77.
-

- 38. K.H.J. Buschow and F.R. De Boer, in “Physics of Magnetism and Magnetic Materials” (Springer US, 2003) p.131.
-

- 39. M. El-Saadawy, J. Magn. Magn. Mater. 219[1] (2000) 69-72.
-

 This Article
This Article
-
2020; 21(3): 378-385
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.378
- Received on Dec 15, 2019
- Revised on Mar 19, 2020
- Accepted on Mar 20, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Yujie Yang
-
Engineering Technology Research Center of Magnetic Materials, School of Physics & Materials Science, Anhui University, Hefei 230601, P. R. China
Tel : +86 551 63861257
Fax: +86 831 63861257 - E-mail: loyalty-yyj@163.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.