- Assessment of the partial and total replacement of feldspar by waste glass on porcelain properties
Marwa Marza Salman* and Hussein Talab Nhabih
Faculty of Materials Engineering, Babylon University, Babylon, Iraq
In this study, soda-lime
silica waste glass was used as partial and total replacement for feldspar in
porcelain. The prepared samples from feldspar, quartz, kaolin and different
percentages of waste glass by powder technology technique were sintered at
1,100 ºC and 1,200 ºC. The tests measured for samples involves
mechanical, physical and thermal properties. A results of these tests indicated
an increasing and decreasing in the samples properties with increasing of waste
glass additive, and that continues to 75% waste glass of feldspar. While X-ray diffraction
analysis indicated a presence of tridymite phase SiO2, mullite phase
2SiO2.3Al2O3 and anorthite phase CaAl2Si2O8
in the sample contained 100% waste glass of feldspar and fired at
1,200 ºC. This study showed possibility of replacement of feldspar used in
porcelain industry by waste glass as flux, and a percent of waste glass
additive, which gives the best properties of porcelain samples, is 75% wt. of
feldspar.
Keywords: soda-lime silica waste glass, porcelain, feldspar, fracture strength, kaolin
Reusing of some materials waste into ceramics manufacture
has been widely studied in the last years in order to economically justify the
great costs related to its manufacture as well as to evade landfilling these
wastes [1-5].
Porcelain products are made in most of world countries and
porcelain's technology is described in many papers
and diverse textbooks [6]. The term porcelain is more
accurately limited to translucent vitreous ware though it is occasionally used
to a variation of vitreous and semi vitreous ware. Porcelain is
a hard, fine-grained, nonporous [7]. A wide-ranging compositions of
triaxial ceramic which are utilized in the
industries of white ware essentially comprise quartz, feldspar
and kaolin. Porcelain shells and porcelain insulators are significant equipment
in the insulation operation of transformer substations and power plants and
supporting wire [8]. Materials of porcelain have high-interest
properties to various industrial applications, such as
high mechanical strength; low thermal conductivity; very low thermal expansion
and excellent thermal shock [9, 10].
One of the materials which use as flux in the industry of
porcelain is feldspar. Feldspar is forming around 60% of
earthly rocks by far the most plentiful group for minerals in
the earth crust. Potassium feldspar, sodium feldspar and mixed feldspars are
offered in most deposits. Feldspars are mainly utilized in
manufacturing applications because their alkali and alumina
content. The term feldspar includes a entire range of materials. More the
products we utilize as a basis in daily life are manufactured
by feldspar: glass for protection, glass for drinking, the
tableware from which we eat, fiberglass for insulation and the shower basins
and floor tiles in our bathrooms. Feldspar can be an essential part in our
daily life [11, 12].
The most common and cheapest form of glass is soda-lime
silica glass, where it forms 90% of glass made. It generally
comprises 5-12% lime, 12-18% soda, and 60-75% silica. Its resistance to thermal
shock and resistance to elevated temperatures are low, and resistance to the
corrosive substances is only fair. For soda-lime glass, the melting temperature
is 1,000 oC while the glass transition temperature is
520-600 oC [13]. A large volumes of waste container glass from
bottle banks produced worldwide are presently earth filled. The container glass
involves primarily sodium, calcium and silicon oxides (denoted to as
soda-lime-silica glass, which can be symbol with SLS glass), where SLS glass
includes primarily sodium, calcium and silicon oxides with other secondary
constituents, such as aluminium oxide (Al2O3) and
magnesium oxide (MgO). SLS waste glass is non-hazardous and its recycling can
significantly reduce the consumption of natural raw materials yielding both economical
and environmental benefits. The chemical composition of SLS glass is alike to
that of natural fluxes utilized in the industry of whitewares such as nepheline
syenite and feldspar [14].
Added glass is expected to act as a flux promoting the
formation of liquid phase and thus reducing the clay body
maturation temperature. Possible commixtures in glass
waste, such as ceramics and stones, can make as filler substance. Additional
interests of the recycled glass in the ceramic manufacturing
can be more practical in nature. The industrial ceramics
sector uses large volumes of materials, therefore large amounts of glass can be
recycled. At the same time, transportation costs can be kept at a minimum since
industries are usually geographically widespread. Furthermore, the reduced
specific heat of glass in conjunction with its low energy demand
for the physico-chemical transformations during firing and
the expected decrease in body maturation temperature can contribute to energy
saving [15]. In 2005, Pontikes Y., et al. investigated possibility of
introducing soda-lime silica scrap glass (SLS glass waste) for ceramics
production, the axes of research were: i) production of porcelain stoneware
tiles by substituting diverse quantities of sodium feldspathic sand and ii)
production of roofing tiles by substituting part of the standard clay mixture
[15]. In 2012 Çagatay K., et al. studied an use of borosilicate glass waste as
a fluxing agent in porcelain bodies [16]. In 2014, Tarnkamol T. and
Guilherme P. S. used soda-lime silica waste glass as partial substitution for
natural fluxes in whiteware formulations may significantly contribute to
continuous development of the glass industries and traditional ceramics [14].
In 2018, Khaled B., et al. studied the effects of waste glass recycling
which derived from broken car glass as partial substitution of potassium
feldspar in porcelain, a results of this study stated an important effects of
sintering temperature and waste glass recycling substitution on the physical
properties [9].
The present study aims to investigate an use effects of
soda-lime silica waste glass as partial and total replacement
for feldspar which uses as flux in porcelain industry
on the different properties for porcelain samples; such as
mechanical, physical and thermal properties; and to determine approximately the
percent of additive waste glass which gives more effect on the properties of porcelain samples.
Consequently, the contribution in a
development of the ceramics industries and an elimination of the large volumes
of waste container glass from the land, and that leads to an economical and
environmental benefits).
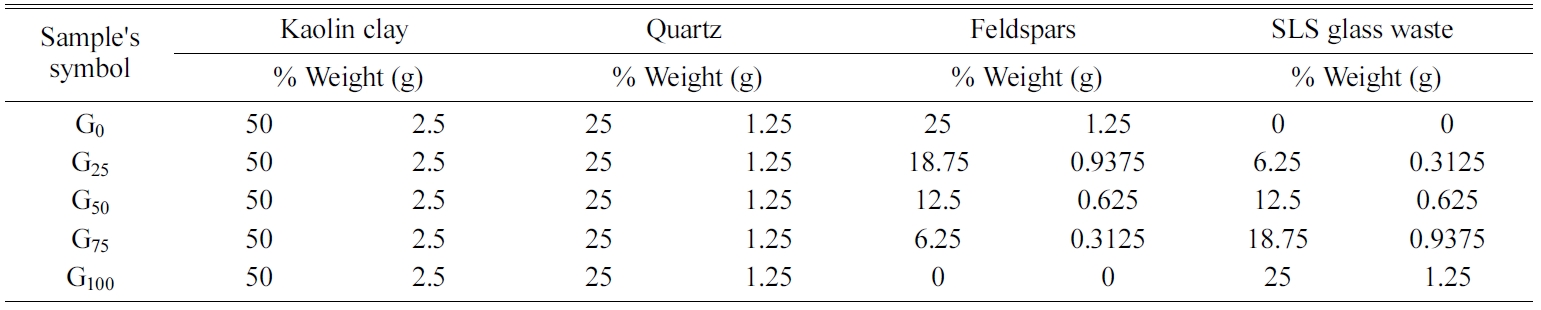
The current study utilizes available raw materials to
produce porcelain samples, where these samples were prepared from a blend of
kaolin clay, quartz as a filler, and feldspar and colorless soda-lime silica
glass waste as fluxing agents by using powder technology.
Materials
preparation
The grinding, sieving, weighting and mixing process were
made for the powders of materials which used in the present
study by use an electrical equipment prepared for that
purpose. Where these powders were mixed according to
the mixing percentages in Table 1 by use the
electrical mixer for 3 h to obtain a homogeneous mixture.
Samples
formation
A steel die with diameter 20 mm and hydraulic uniaxial
pressing machine were used in the formation of porcelain samples by using a semi
dry pressing technique and a pressure of 60 MPa, then temperature 110 ºC
was selected to dry the formed samples for five hours to eliminate the wetness
from the samples and temperatures 1,100 ºC and 1,200 ºC were selected
to sinter it with heating rate 5 ºC/min and soaking period 2 h, the
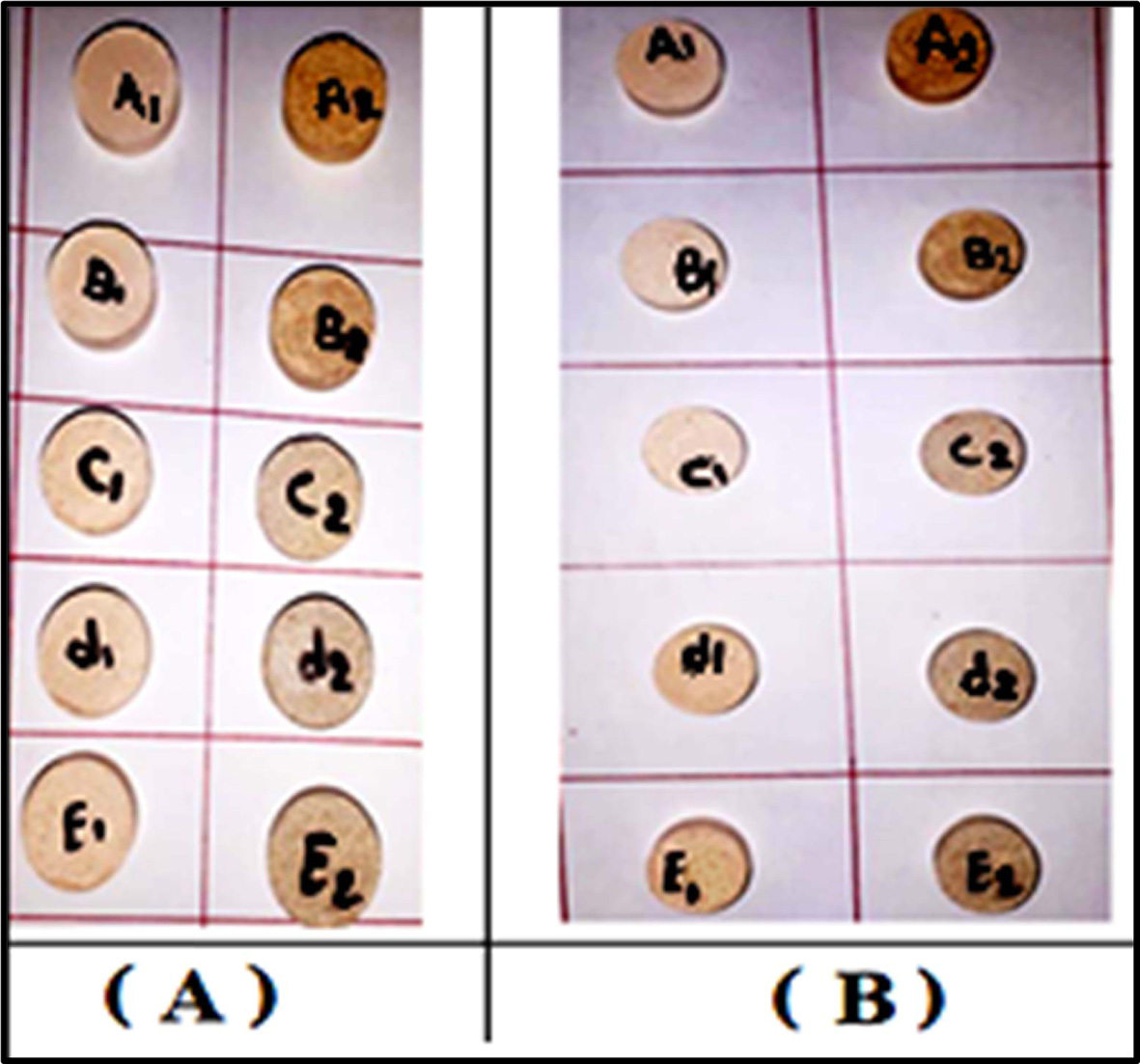
cooling of samples was finished in the furnace. Fig. 1 displays the sintered
samples.
Tests
The tests which were made for porcelain samples produced
were included the mechanical properties (hardness and fracture strength), the
physical properties (linear shrinkage, density, porosity and XRD) and thermal
properties (DTA), which were done as the following:
Mechanical
properties
Depending on ASTM standard (C 773-88) [17], fracture
strength of the samples was tested using general testing
machine. Fracture strength was determined from the following formula.
бf = L / CA (1)
where бf is a fracture strength
(MPa); L is an applied load up to fracture (N); CA is a cross
section area mm2.
Apparatus of digital microvickers hardness tester (TH-717) was utilized
to test Vickers hardness of porcelain samples according to ASTM standard C1327-90 by
use Vickers indentation manner by 9 kg load applied through 10 seconds
on a surfaces of the specimens. The equation
below was utilized to calculate
Vickers hardness values of porcelain samples [18].
HV = 1.854 × F/ d2 (2)
where HV is vickers hardness of the
samples (kg/mm2); F is an indentation load (kg); d is a half of the
indentation diagonal (mm).
Physical
properties
A linear shrinkage on firing of porcelain samples was
evaluated according to ASTM standard C326 by use the equation below [19].
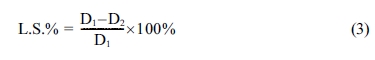
where (L.S) is a linear shrinkage of
porcelain samples; D1 and D2 are the external diameter
for the samples before and after sintering process respectively.
Archimedes technique was utilized to measure the density
and porosity of porcelain samples which were measured according to (ASTM
C373-88) standard, and the formula below was utilized to find a bulk density of
porcelain samples [20, 21].

where D is the bulk density of
porcelain samples (g/cm3); Dwater is the water's
density (g/cm3); MD is the sample's dry mass (g); MP
is the sample's suspended mass (g); MS is the sample's
water-saturated mass (g).
Also the formula below was utilized to find an apparent
porosity of porcelain samples.

where Po = the percent of
apparent porosity of porcelain samples.
X-ray diffraction test was made for a powder of porcelain
specimens to detect a crystalline phases developed in porcelain samples
produced. A mortar and pestle were used to grind porcelain samples to get the
fine powder of samples which was tested by X-ray diffraction. A (SHIMADZU XRD –
6000) machine was utilized in this test to obtain the X-ray diffraction
patterns. An anode of Cu with voltage [40 kv] and current [30 mA] was utilized
to obtain the incessant scan mode using range (θ-2θ) as (20o - 70o),
a speed of the scan was (7o (θ)/min) and a size of the step was (2θ
=0.0200o)
Differential
thermal analysis (DTA)
Differential thermal analysis (DTA) was made to examine
the thermal changes for porcelain samples with and without an addition of the
waste glass for it. A fine powder of porcelain samples produced was used in
this test. Temperature range 0-800 oC was utilized to perform the
DTA analysis by the heating rate 5 oC/min. DTA-50 apparatus linked to
a program and control unit which shows a result of this test.
|
Fig. 1 The sintered samples: a group (A) at (1,100 ºC) and a group (B) at (1200 ºC). |
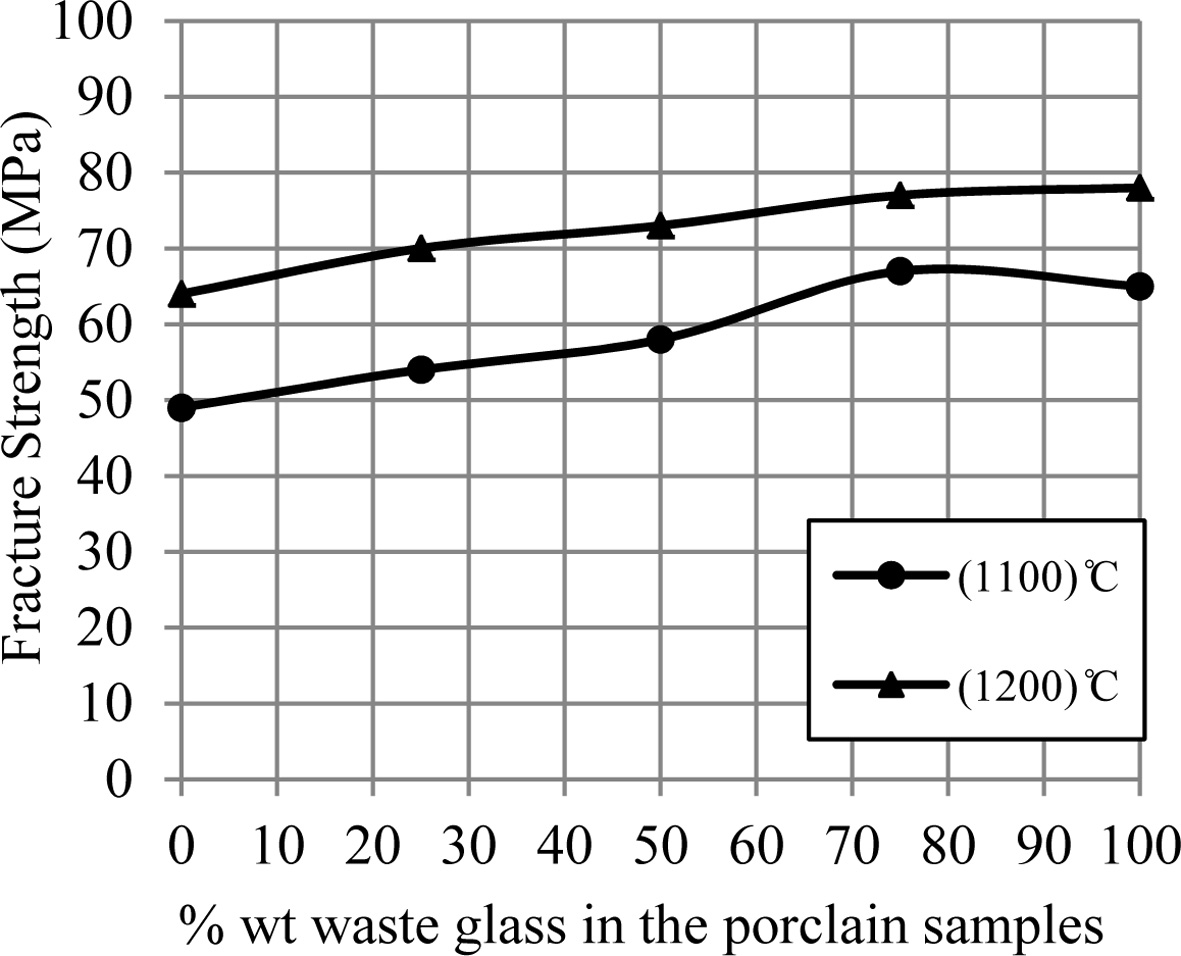
Fig. 2 displays the fracture strength for porcelain
samples. It can be showed that an increase of the fracture strength occurs by
an increase of the additive waste glass percentage. So the
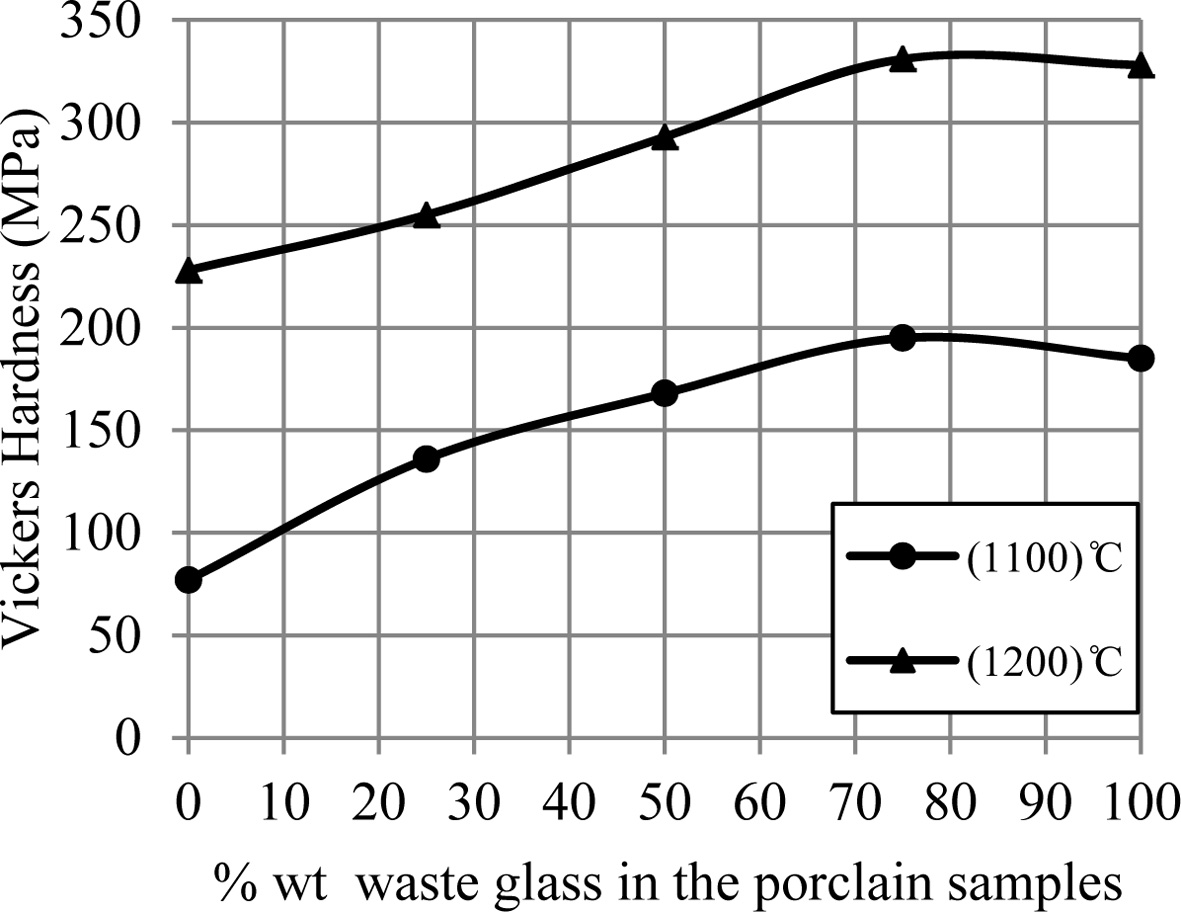
samples' hardness increases by an increase of the additive waste glass
percentage, which is displayed in Fig. 3. This improvement in the mechanical
properties for porcelain specimens produced after an
addition of the waste glass for them was happened because of a formation of the
glassy phase in a matrix of porcelain specimens as a result of an existence of
the great quantity of the diverse fluxes (such as CaO, K2O and Na2O)
within a composition of the waste glass added, that leads to the decrease in
the pores among the substance's particles and the increase in the bond among them
that forms the more-rigid network,
consequently that improves the mechanical strength for porcelain samples [9,
15, 22, 23].
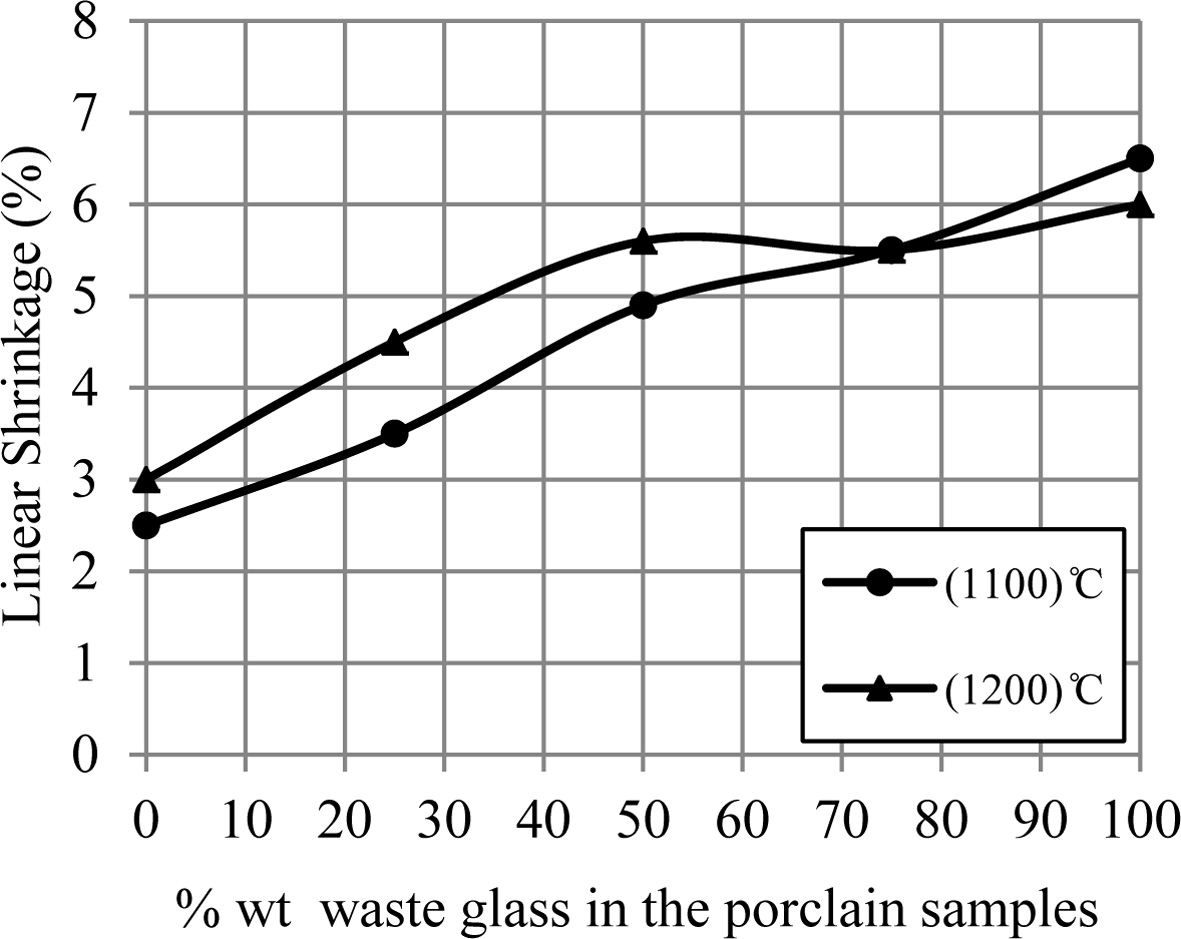
While Fig. 4 displays the linear shrinkage on firing for
porcelain samples. Each one of porcelain samples showed a linear shrinkage
within the range 2.5-6.5% for temperature 1,100 ºC and 1,200 ºC, where the
linear shrinkage values for porcelain specimens raise with an increase of the percentage
of additive waste glass. The samples at temperature 1,200 ºC and until 75%
wt. waste glass were more shrinkage than that at 1,100 ºC due to form of a
great amount of the liquid phase which fills the voids among the particle of
sample, that leads to decrease the total volume of sample (i.e. increase the
linear shrinkage for the samples) [24, 25]. For the samples with 75% waste
glass and up, an important reduction happened in the linear shrinkage values at
1,200 ºC which were a lower than that at 1,100 ºC. Usually, the
existence of liquid phase eases the sintering process of the samples, but a
presence of a great quantity of the low-density liquid phase causes the
swelling in porcelain specimens as happened in this state [9, 26, 27].
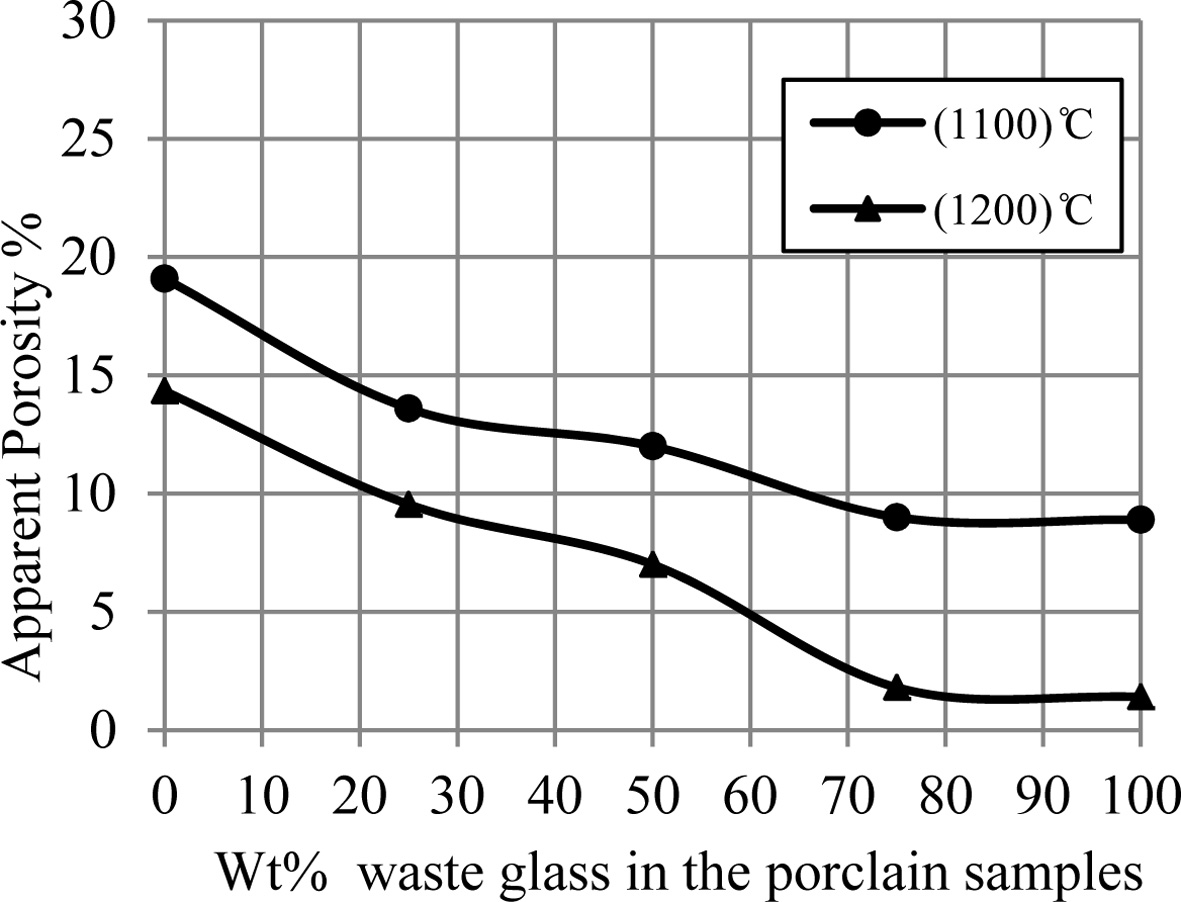
Fig. 5 displays an influence of the additive percentage
of waste glass on an apparent porosity of porcelain specimens. It can be showed
from this figure the decreasing in the porosity of samples with an increase of
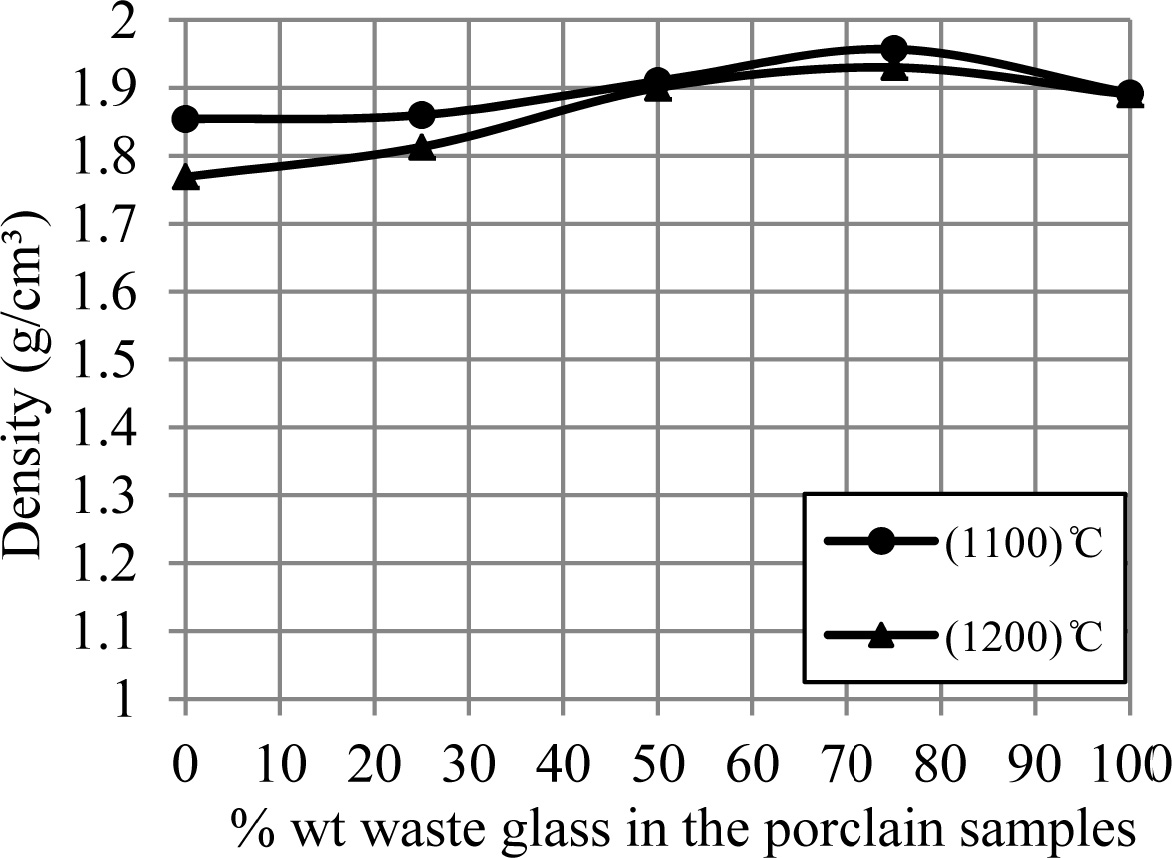
the additive percentage of waste glass. Whereas Fig. 6 displays an influence of
the additive percentage of waste glass on the bulk density of porcelain
specimens, where an increase of the additive percentage of waste glass leaded
to increase the density of samples. These increasing and decreasing in the
density and porosity of samples respectively can be happened because of the
liquid phase formed in a matrix of porcelain specimens for an
existence of the great quantity of the diverse fluxes (such as
CaO, K2O and Na2O) within a composition of the waste
glass added, which lead to the appearance of a large
amount of liquid phase that fills the voids among the
substance's particles and leads to the decrease in the pores in the sample
structure [15, 28], and because of a conversion of the remaining open porosity
to closed porosity [9, 29].
This increase in the density of samples continues to the
percent for waste glass 75% wt. of feldspar, after this percent the state is
contrary (i.e. the density of samples decreased), and addition to the bulk
density of samples at 1,200 ºC was a less than that at 1,100 ºC. This
is a result of the existence of a great amount of low-density
liquid phase leads to a swelling of porcelain specimens
[26, 27], and a result of the liquid phase formed at temperature upper than
1,200 ºC facilitates a liberation of the gas locked in the closed pores,
that causes a creation of the new open porosity [9, 30].
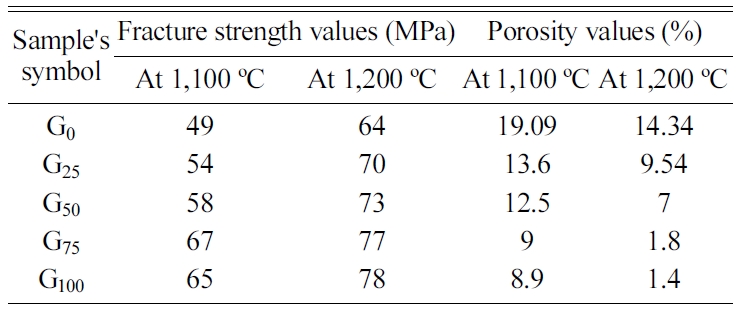
Table 2 displays the fracture strength and porosity values
of porcelain samples for the comparison between these
properties.
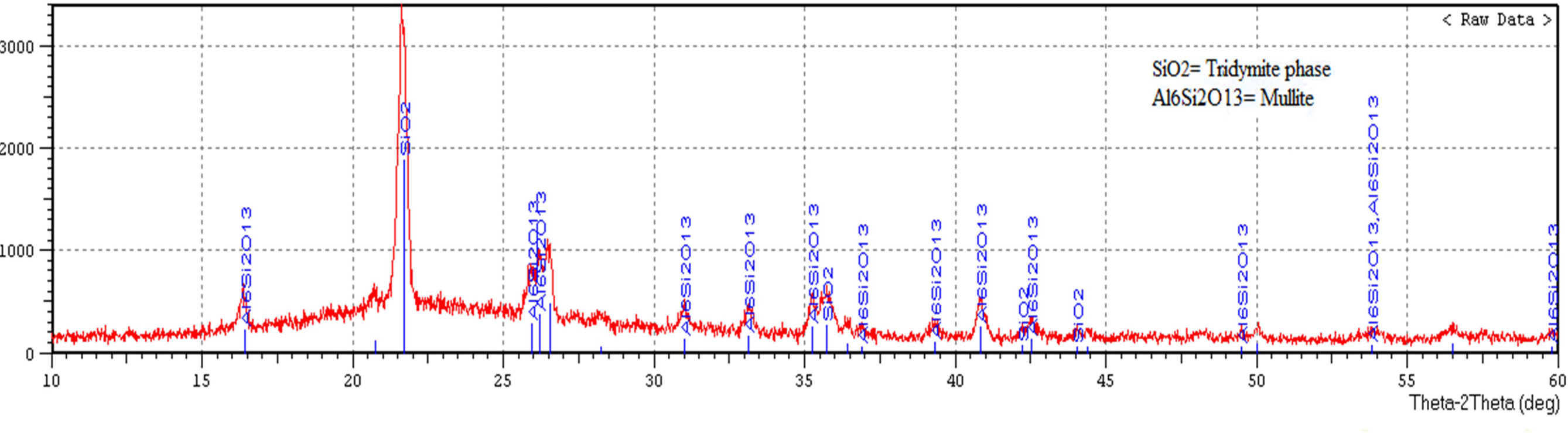
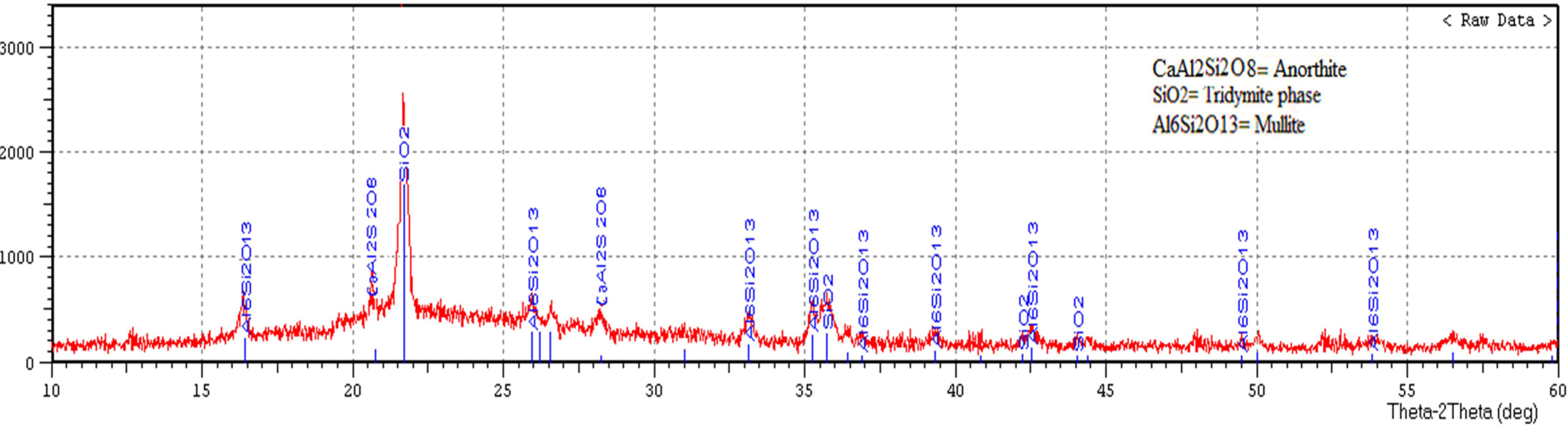
Fig. 7 displays X-ray diffraction pattern of porcelain
specimen produced without the addition of waste glass and fired at
(1,200 ºC), while Fig. 8 displays X-ray diffraction pattern of porcelain
specimen produced by the addition of waste glass 100% wt. of feldspar and fired
at (1,200 ºC). The main difference between these figures is an existence
of anorthite (CaAl2Si2O8) in Fig. 8, anorthite
is a rare phase in porcelain and its crystallization within a composition of
porcelain sample contained on the waste glass is a consequence of the existence
of (CaO) in the powder of soda-lime glass. Another difference is an existence
of mullite (2SiO2·3Al2O3)
in Fig. 7 more than that in Fig. 8 as a result of the utilization of soda-lime glass as a substitute of feldspar, which leads to an existence of the
glass phase composition, as well as
the low aluminum content in a
composition of the sample contained on the waste glass. The highest peak in both the two patterns represents another crystalline phase which is tridymite
phase (SiO2). The peak height proved the presence of a more amount of the tridymite phase in Fig. 7. These
differences between porcelain
samples produced in this study represent as
a peculiarity of the employment of soda-lime glass waste as a substitute of feldspar. This analysis agrees with the
way suggested by [9, 31, 32].
Finally, the (DTA) curve registered through heating for
porcelain sample produced without the addition of waste glass and fired at
(1,200 ºC) is exposed in Fig. 9, while Fig. 10 displays the (DTA) curve
for porcelain sample produced with the addition of waste glass 100% wt.
of feldspar and fired at (1,200 ºC). There was a clear difference between
the two curves after temperature (600 ºC), which
approximates from the glass transition temperature of soda-lime silica glass,
which be in the range 520-600 ºC [13]. An influence of an use of the waste
glass as a replacement of feldspar on the thermal behavior for porcelain samples
in this test was due to an existence of the great quantity of the diverse
fluxes (such as CaO, K2O and Na2O) within a composition
of the waste glass added, these fluxes facilitate the
sintering process for porcelain samples, and thus facilitating the formation
of phases since its glass transition temperature is low [28,
33, 34, 35].
|
Fig. 2 A change of fracture strength of porcelain samples with increase the percent of additive waste glass. |
|
Fig. 3 A change of hardness of porcelain samples with increase the percent of additive waste glass. |
|
Fig. 4 A change of linear shrinkage of porcelain samples with increase the percent of additive waste glass. |
|
Fig. 5 A change of apparent porosity of porcelain samples with increase the percent of additive waste glass. |
|
Fig. 6 A change of bulk density of porcelain samples with increase the percent of additive waste glass. |
|
Fig. 7 X-ray diffraction pattern of porcelain specimen produced without the addition of waste glass and fired at (1,200 ºC). |
|
Fig. 8 X-ray diffraction pattern of porcelain specimen produced by the addition of waste glass 100% wt. of feldspar and fired at (1,200 ºC). |
|
Fig. 9 The (DTA) curve for porcelain sample produced without the addition of waste glass and fired at (1,200 oC). |
|
Fig. 10 The (DTA) curve for porcelain sample produced with the addition of waste glass 100% wt. of feldspar and fired at (1,200 ºC). |
This study shows a possibility of replacement of the
feldspar used in porcelain industry with soda-lime waste glass as a flux, and
the percent of waste glass additive which gives the best properties of
porcelain samples is 75% wt. of feldspar. The linear shrinkage on firing and
density of porcelain specimens raise by an increase of
the additive percentage of waste glass. In the samples with 75% wt. waste glass
and up, a significant reduction in the linear shrinkage
values at 1,200 ºC to compare with that at 1,100 ºC, and the increase
in the density of samples converts to a decrease. While, the porosity of
specimens reduces by an increase of the additive percentage of waste glass. In
addition, the fracture strength and hardness of porcelain specimens increase by
an increase of the additive percentage of waste glass to them. X-ray
diffraction analysis proves an existence of crystalline phases in porcelain
matrix, which refers tridymite phase (SiO2) and mullite phase (2SiO2·3Al2O3)
for both the two states (i.e. with and without use of waste glass in porcelain
samples), so it shows a present of anorthite phase (CaAl2Si2O8)
in porcelain sample contained 100% waste glass of feldspar
and fired at 1,200 ºC. While, the difference between the DTA curves for
porcelain samples ensures that the use of waste glass as the replacement of
feldspar affects on the thermal behavior for these samples. Then, the
samples with 75% wt. waste glass give the best effect on the
properties of porcelain samples.
- 1. H.R. Fernandes, D.U. Tulyaganov, and J.M.F. Ferreira, J. Adv. Appl. Ceram. 108[1] (2009) 9-13.
-

- 2. A. Karamanov, J. Adv. Appl. Ceram. 108[1] (2009) 14-21.
-

- 3. I. Ponsot, R. Detsch, AR. Boccaccini, and E. Bernardo, J. Adv. Appl. Ceram. 114[Suppl. 1] (2015) S17-S25.
-

- 4. P.R. Monich, D. Desideri, and E. Bernardo, J. Adv. Appl. Ceram. 118[6] (2019) 366-371.
-

- 5. M.L. Antunes, A. Botignon de Sá, P.S. Oliveira, and E.C. Range, Cerâmica 65[Suppl.1] (2019) 1-6.
-

- 6. A. Karamanov, E. Karamanova, A. Ferrari, F. Ferrante, and M. Pelino, J. Ceram. Int. 32[7] (2006) 727-732.
-

- 7. A. Akkus and T. Boyraz, J. Ceram. Process. Res. 20[1] (2019) 54-58.
-

- 8. S. Bhattacharyya, S.K. Das, and N.K. Mitra, J. Bull. Mater. Sci. 28[5] (2005) 445-452.
-

- 9. B. Khaled, B. Abdelhafid, B. Hocine, and R. Maximina, Bol. Soc. Esp. Cerám. Vidr. 58[1] (2019) 28-37.
-

- 10. S. Wattanasiriwech and D. Wattanasiriwech, J. Ceram. Process. Res. 20[6] (2019) 643-648.
-

- 11. S. Radomír and V. Lucie, Int. J. Eng. Sci. 4[10] (2014) 49.
- 12. N. Ediza and A. Yurdakulb, J. Ceram. Process. Res. 10[6] (2009) 758-769.
- 13. S. Frank, in “The Corning Museum of Glass Education Dept. Education Coordinator” (The Corning Museum of Glass, 1998) p.82.
- 14. T. Tarnkamol and P.S. Guilherme, International Conference on Geology of Thailand: Towards Sustainable Development and Sufficiency Economy, (2014) 276.
- 15. Y. Pontikes, A. Christogerou, and G. Angelopoulos, Article in Glass Technology, 78 (2005) 54.
- 16. Ç. Koca, N. Karakus, N. Toplan, and H. Toplan, J. Ceram. Process. Res. 13[6] (2012) 693-698.
- 17. ASTM, C 773-88 (1988) 245.
- 18. M. Roy. The University of British Columbia, (2010) 89.
- 19. W.D. Kingery, H.K. Bowen, and D.R. Uhlmanm, in “Intro- duction to Ceramics, 2nd Edition” (John Wiley and Sons, 1975) p.1032.
- 20. G. Kaya, Ceram. Int. 39[1] (2013) 511-517.
-

- 21. ASTM, C 373 (1988) 195.
- 22. K. Nuntaporn, C. Benya, B. Warunee, and C. Parinya, J. Met., Mat. Min. 29[3] (2019) 71-75.
- 23. Y. Dong, X. Feng, Y. Ding, X. Liu, and G. Meng, J. Alloy. Compo. 460 (2008) 599-606.
-

- 24. O. Watanabe and S. Kato, in “Environmental Issues and Waste Management Technologies in the Ceramic and Nuclear Industries VI” (Am. Ceram. Soc., 2001) p.113-123.
- 25. B. Achiou, H. Elomari, M. Ouammou, A. Albizane, J. Bennazha, A. Aaddane, S.A. Younssi, and I. El Amrani El Hassani, J. Mater. Environ. Sci. 9[3] (2018) 1013-1021.
-

- 26. K. Kim and J. Hwang, J. Ceram. Int. 41[5] (2015) 7097-7102.
-

- 27. E. Bernardo and P.A. Bingham, J. Adv. Appl. Ceram. 110[1] (2011) 41-48.
-

- 28. I.M. Bakr, J. Adv. Appl. Ceram. 104[5] (2005) 243-248.
-

- 29. A. Ahmet and B. Tahsin, J. Ceram. Process. Res. 20[1] (2019) 54-58.
-

- 30. Y. Iqbal and W.E. Lee, J. Am. Ceram. Soc., 83[12] (2000) 3121-3127.
-

- 31. R.B. Saulo and P.B. Carlos, Mat. Res. 8[1] (2005) 67.
- 32. H.R. Fernandes and J.M.F. Ferreira, J. Eur. Ceram. Soc. 27[16] (2007) 4657-4663.
-

- 33. M. Heraiz, F. Sahnoune, H. Belhouchet, and N. Saheb, J. Optoelectron. Adv. Mater. 15[11-12] (2013) 1263-1267.
- 34. A.J. Souza, B.C.A. Pinheiro, and J.N.F. Holanda, J. Environ. Manage. 91[3] (2010) 685-689.
-

- 35. S.Y.R. Lopez, J.S. Rodriguez, and S.S. Sueyoshi, J. Ceram. Process. Res. 12[3] (2011) 228-232.
 This Article
This Article
-
2020; 21(3): 371-377
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.371
- Received on Nov 29, 2019
- Revised on Mar 21, 2020
- Accepted on Mar 24, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- Marwa Marza Salman
-
Faculty of Materials Engineering, Babylon University, Babylon, Iraq
Tel : +964-7809430850
Fax: +964-7809430850 - E-mail: marwamarzas@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.