- Optimization of low temperature hydrogen sensor using nano ceramic-particles for use in hybrid electric vehicles
J. Niresha,*, N. Archanab, S. Neelakrishnanc, V. M. Sivakumard and D. S. Dharune
aAssistant Professor, Department of Automobile Engineering, PSG College of Technology, Coimbatore, India
bAssistant Professor, Department of EEE, PSG College of Technology, Coimbatore, India
cProfessor, Department of Automobile Engineering, PSG College of Technology, Coimbatore, India
dAssociate Professor, Department of Chemical Engineering, Coimbatore Institute of Technology, Coimbatore, India
ePG Scholar, Department of Automobile Engineering, PSG College of Technology, Coimbatore, India
Hydrogen sensing in automobile
application is the need of the hour as fuel cell based hybrid electric vehicle
is developing at a faster pace. Nickel ferrite (NiFe2O4)
nanoparticles embedded reduced graphene oxide (rGO) prepared by a facile
hydrothermal thermal process was employed as a chemiresistive sensor for the
detection of H2 gas. To study the morphological and structural
features the synthesized samples were characterized by transmission electron
microscopy (TEM), X-Ray diffraction and Raman Spectroscopy. 50 nm cube shaped
NiFe2O4 nanoparticles were found to be distributed on few
layered rGO nanosheets. The electrical conductivity of pristine n type NiFe2O4
and NiFe2O4/rGO were tested at various operating
temperatures. Hydrogen sensing characteristics were measured by forming a thick
film of the synthesized nanocomposite paste on an alumina substrate. Sensing
results showed that NiFe2O4/ 1% rGO showed the maximum
response at an optimum temperature of 80 ºC towards 200 ppm of hydrogen gas
among four variants. Integration of NiFe2O4 nanoparticles
on rGO nanosheets has markedly enhanced the conductivity of the nanocomposite.
The sensor showed a lower detection limit of 8 ppm with two linear ranges from
30-90 ppm and 100 to 700 ppm. Further the sensor was tested for its selectivity
and stability.
Keywords: Hydrogen sensor, Nanoparticles, Chemiresistive, Electric Vehicles
With the growing population, the need for alternate green
source of energy has become inevitable due to the depletion of fossil fuels.
Among the various natural fuel sources, hydrogen is considered
as the next generation energy source especially in the
automobile industry [1, 2]. It has found its prominence due to extraordinary
properties such as high energy content, no release of harmful gases
on combustion and low molecular weight [3, 4].
Nevertheless, H2 is highly volatile and flammable
in nature. A small leak into the atmosphere could cause explosion. Since H2
being an odourless and a colorless gas, it cannot be detected by human senses,
perhaps special sensors are required. In order to safely exploit the benefits
of H2 energy, it is inevitable to develop sensors that can detect H2
specifically from a mixture of gas. Among the various hydrogen sensors
available such as electrochemical sensors, gasochromic sensors, fiber optic
etc, chemiresistive sensors seem to be a good choice [5, 6]. Recently, it is
known from the literature that carbon based materials can detect H2
at room temperature and also they tend to shown high conductivity with the
adsorption of H2 on its network [7, 8]. Carbon nanomaterials such as
graphene based gas sensors have attracted huge attention due to their stunning
electrical and electronic properties [9-12]. Graphene has a high theoretical
surface area (2630 m2g−1) and electrical conductivity (106
Scm−1) which makes it a promising material for H2 sensing
[13]. A derivative of graphene which can be easily synthesized by chemical
route is called reduced graphene oxide (rGO). Most
graphene based gas sensors are increasingly being
employed owing to three reasons [9]:
1. Its abundant defects and chemical groups
facilitate gas adsorption
2. The chemical and electrical properties of rGO
are highly tuneable by composite formation
3. Compared to its precursor Graphene oxide, rGO
has high charge transfer rate.
Though numerous rGO based H2 sensors have been
developed, pristine graphene sensors suffer from low chemisorption of target
gas molecules because of a few dangling bonds present on their surface. In a
graphene stack, due to the van der Waals and π-π interactions among individual
graphene there is high tendency for aggregation when its dispersion is dried.
Integration of nanostructures with gas-sensing ability into graphene sheets
inhibits agglomeration in graphene and also aids in good
distribution of nanostructures. Thus, the effective surface
area available for the gas interaction increases several fold [14].
To date, quite a few rGO based composite materials have
been synthesized by incorporation of metal nano- particles for
better sensing properties [15-19]. However, the
glitches with the traditional Pt, Pd nanoparticles is that they exhibit very
high recovery time due to the formation of hybrids on hydrogen exposure. On the
other hand, semiconducting metal oxides such as SnO2, ZnO, CoO, TiO2,
Fe2O3 are also being used for gas sensing applications
due to their easy implementation, good reliability and low cost in real time
systems [20-22]. Gas sensing is a surface phenomenon where the adsorption of
gases can alter the conductivity of the metal oxides. Depending on the
operating temperature and the morphology of the metal oxide nanoparticles,
distribution of various oxygen species such as O-,
O2-
and O2-
differs [23]. Han et al fabricated functionalized RGO that demonstrates
excellent sensitivity to H2S gas. The functionalization process
used GO suspensions mixed with AgNO3, NaOH, and DI water
[24]. Shivi et al. studied the Surface Enhanced Raman Spectroscopy of reduced
graphene oxide modified with silver nano- particles. They found that the D peak,
G peak and 2D peak intensity of Raman spectra has been amplified many times
with silver nanoparticles as well as silver nanoparticles
intercalate between the layers of graphene oxide,
therefore it is also helpful in reduction of graphene oxide [25].
Although metal oxide nanoparticles increase the sensitivity,
the selectivity is dependent on dopants. However from literature it has been
understood that controlling the shape of the nanocrystallites can effectively
change energy of different adsorption sites thereby adsorbing gases at a lower
temperature. Thus, the existence of large surface to volume ratio in metal oxides
yield better performance [26]. Nevertheless, metal oxides as
individual sensing units detect hydrogen only at elevated temperatures due to
wide band gap and high electrical resistance in the range of kilo to mega ohms.
Multi component systems such as spinel nanostructures with
general formula of AB2O4 are noteworthy materials
to detect both oxidizing and reducing gases. Among the spinel type structures,
ceramic NiFe2O4 finds its application in sensing gases
like Cl2, NH3 etc, dye degradation,
Li-ion batteries and magnetic hyperthermiac [27-32]. It exhibits excellent stability, electrochemical activity and
electrical conductivity that arises due to thermal activation of electrons or
holes in the ionic lattice. Usually, the charge transfer occurs through hopping process between the cations of different
valences at a low activation energy. The oxygen atom of
NiFe2O4
occupies the FCC position and the cations Fe3+ and Ni2+
occupy the tetrahedral and octahedral positions. During the synthesis of NiFe2O4 by hydrothermal process, Fe2+ ions always forms with Fe3+.
In order to compensate the charge imbalance, positive oxygen vacancies are
created. The electrons hop between the cations of different valences in O-
sites providing the necessary active metal centres for adsorption of analyte
gas molecules thereby facilitating the catalytic activity [32]. When these
spinel nanostructures are supported on graphene carbon network, they are known
to exhibit improved stability as well as electrochemical response. For
instance, compared to pristine NiFe2O4, NiFe2O4/rGO showed higher HER activity [33]. Liu et al.
reported that mixing of ZnFe2O4
into graphene can lower the operating temperature of acetone sensors [34]. Lin
et al showed that graphene/CuO composites can detect low level of NO2 at room temperature [35].
The performance improvement of these
nanocomposites are attributed to the presence of multiple redox states which
aid in the enhanced catalytic activity. Also, the synergistic effect between
graphene and spinels adds novel properties to the hybrid. The detection of H2
gas employing NiFe2O4 and rGO have not be reported
till date. Herein, we report a facile synthesis of NiFe2O4/rGO
by in-situ hydrothermal method. The synthesized material was used for the
fabrication of a chemiresistive H2 sensor with an improvement in
response, recovery time and reduction in operating temperature.
Reagents
Graphite powder (Alfa Aesar), NaOH (HiMedia), NaNO3
(HiMedia),
H2SO4 (Merck), potassium permanganate (KMnO4) (Merck), H2O2
(Merck), and chemicals such as HCl, NiCl2 and FeCl3 were
purchased from Loba-Chemie. All the solutions were dissolved in dissolved water and used for
the studies
Preparation
of graphene oxide
Graphene oxide was synthesized by the modified Hummer’s
method, a well-established protocol to mass produce
graphene oxide [36]. In short, 1.25 g of graphite powder was
added to a precooled mixture of 25 mL H2SO4 and 1.25 g
NaNO3. To it, 3.75 g of KMnO4, the oxidizing agent is
added slowly under vigorous stirring. After addition the solution is stirred for
2 h at 35 oC. Then 50 mL of water was added to the solution and the temperature was raised
to 95 oC and maintained for 15 min. Subsequently the solution was
quenched with water and brought to room
temperature. 10 mL of 30% H2O2 was added slowly and the solution
was stirred for 10 min. The resulting solution was washed with 5% HCl to remove
the metal ions and further with distilled water to raise the pH to 7.
Lastly, washed solution was dried in an hot air oven at 90 oC for 8
h to get powders of graphite oxide. To obtain graphene oxide, the graphite oxide powders were dissolved in water and
ultrasonicated for 30 min.
Preparation of NiFe2O4/rGO
0.1 M NiCl2 and 0.2 M of FeCl3 were
mixed in 50 mL distilled water. To it 0.01 M of NaOH and different
concentration of GO (0.5%, 1%, 1.5% and 2%) was added and the solution was
stirred well in a magnetic stirrer at 300 rpm for 10 min. The mixture was then
transferred to a teflon lined autoclave and heated at 180 ºC
for 12 h. The resting solution was centrifuged, washed with water and dried
under vacuum. The powders were then subjected to calcination
at 400 ºC.
Material
characterisation
The morphology of the nanocomposite was studied using
Transmission electron microscope (TEM) from JEOL JEM 2100. The X-ray
diffraction (XRD) pattern was recorded from Bruker DS Advance with Cu Kα as
radiation source with a wavelength of 1.54 Å. The Laser Raman spectroscopy was
done using Renishaw inVia fitted with 785 nm laser.
To fabricate the sensor, a thick film of the composite
paste was made on a clean alumina substrate having copper electrical contacts.
The paste was prepared by mixing sufficient amount of ethanol to the
synthesized powder. A film of 50 µm thickness was coated on the substrate.
The fabricated sensor was then placed in a test chamber at appropriate
temperature and other environmental conditions. As a known
quantity of H2 gas is passed, change in voltage with across a
resistor connected in series with the fabricated resistor was recorded using a
Keithley data acquisition module KUSB-3100 interfaced with a computer. The
sensor performance is given as Ra/Rg ratio where Ra and Rg are the resistances
in the presence of air and saturated H2 gas respectively. The time
taken by the sensor to reach 90% of the total resistance change in case of
adsorption is determined as response time and in case of desorption the time
taken for the sensor to reach 10% of the total resistance is determined as recovery
time. All the fabricated sensors were tested following the above procedure at
varying temperatures of 20 to 200 ºC with an interval of 20 ºC.
Material
characterisation
The NiFe2O4/rGO based H2 sensor
was constructed based on the electrostatic interaction between the moieties.
The oxygen functional groups present in the graphene oxide increases the
hydrophilicity of the material. The NiFe2O4 were prepared
by the in-situ co-precipitation of NiCl2 and FeCl3 in the
presence of GO at elevated temperatures. During the process, GO loses most of
its functional groups and NiFe2O4 are anchored on the rGO
matrix through an electrostatic interaction between the
spare functional groups of rGO and NiFe2O4 nanoparticles.
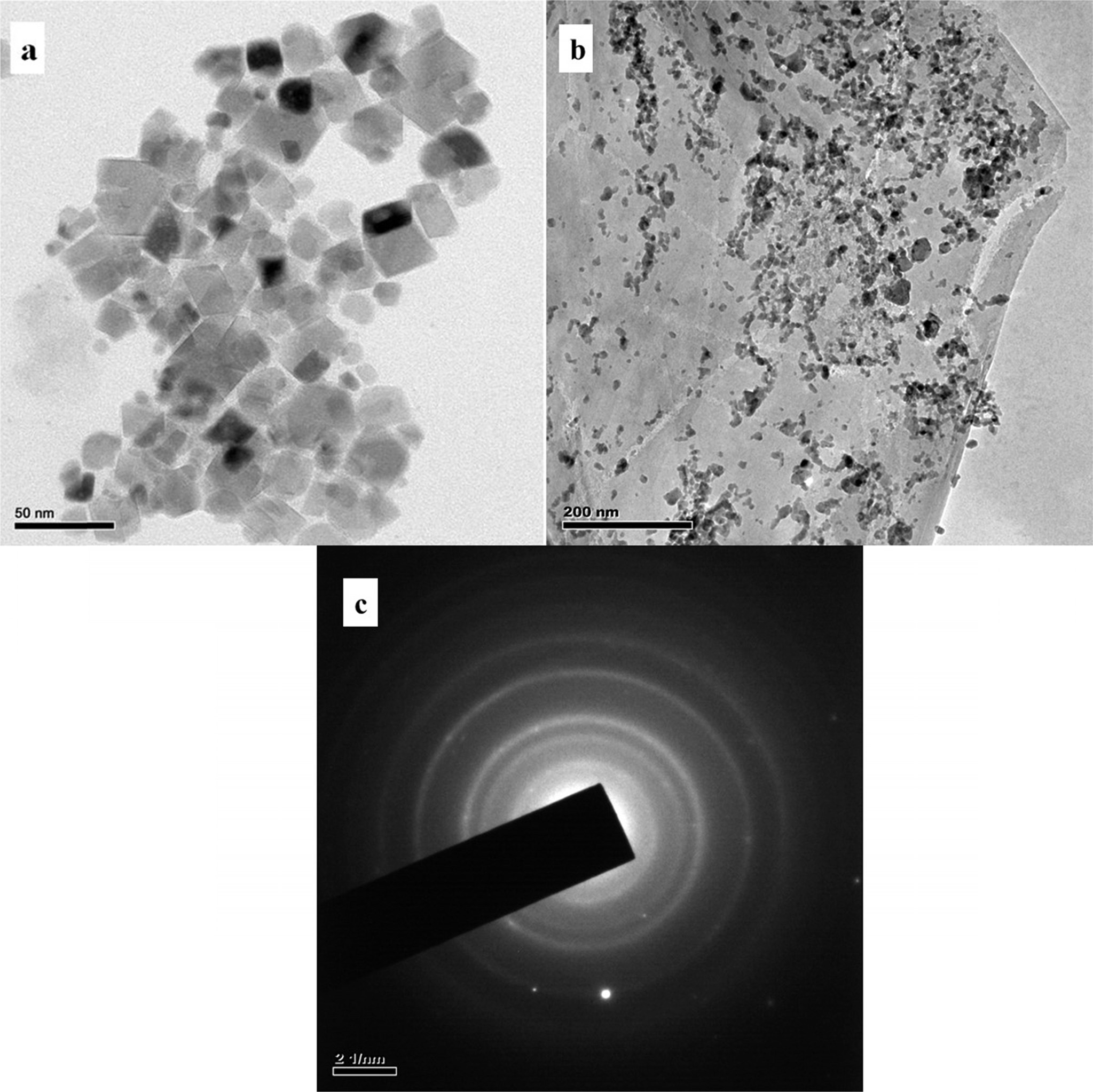
Fig. 1 shows the TEM images of NiFe2O4 and NiFe2O4/rGO
respectively. NiFe2O4 produced by
co-deposition showed a cube like morphology with an average particle size of 55
nm. Fig. 1(b) clearly depicts a good distribution of NiFe2O4nanoparticles
anchored on sheets like structure of rGO. Also rGO was found to be transparent
with few layers and high surface area. Fig. 1(c) shows the SAED pattern of the
composite.
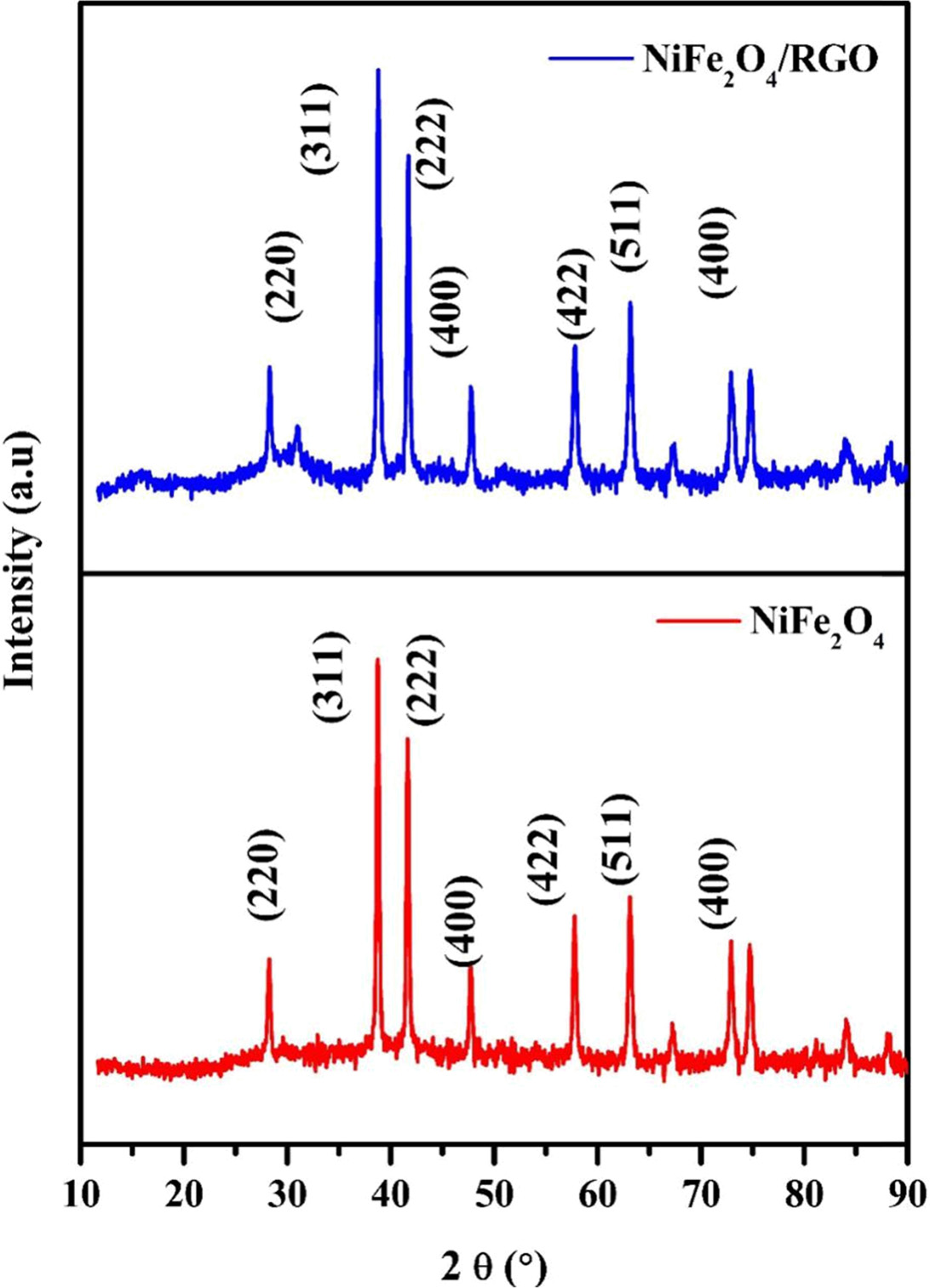
Fig. 2 depicts the XRD pattern of
pristine NiFe2O4 and NiFe2O4/rGO
composite. The sharp peaks indicate the crystalline nature of the
nanocomposite. Both the materials exhibit sharp peaks which could be indexed to
(220), (311), (222), (400), (422), (511) and (440) planes of the cubic and face
centered structure of NiFe2O4. The values were in
accordance with the JCPDS number 54-0964.
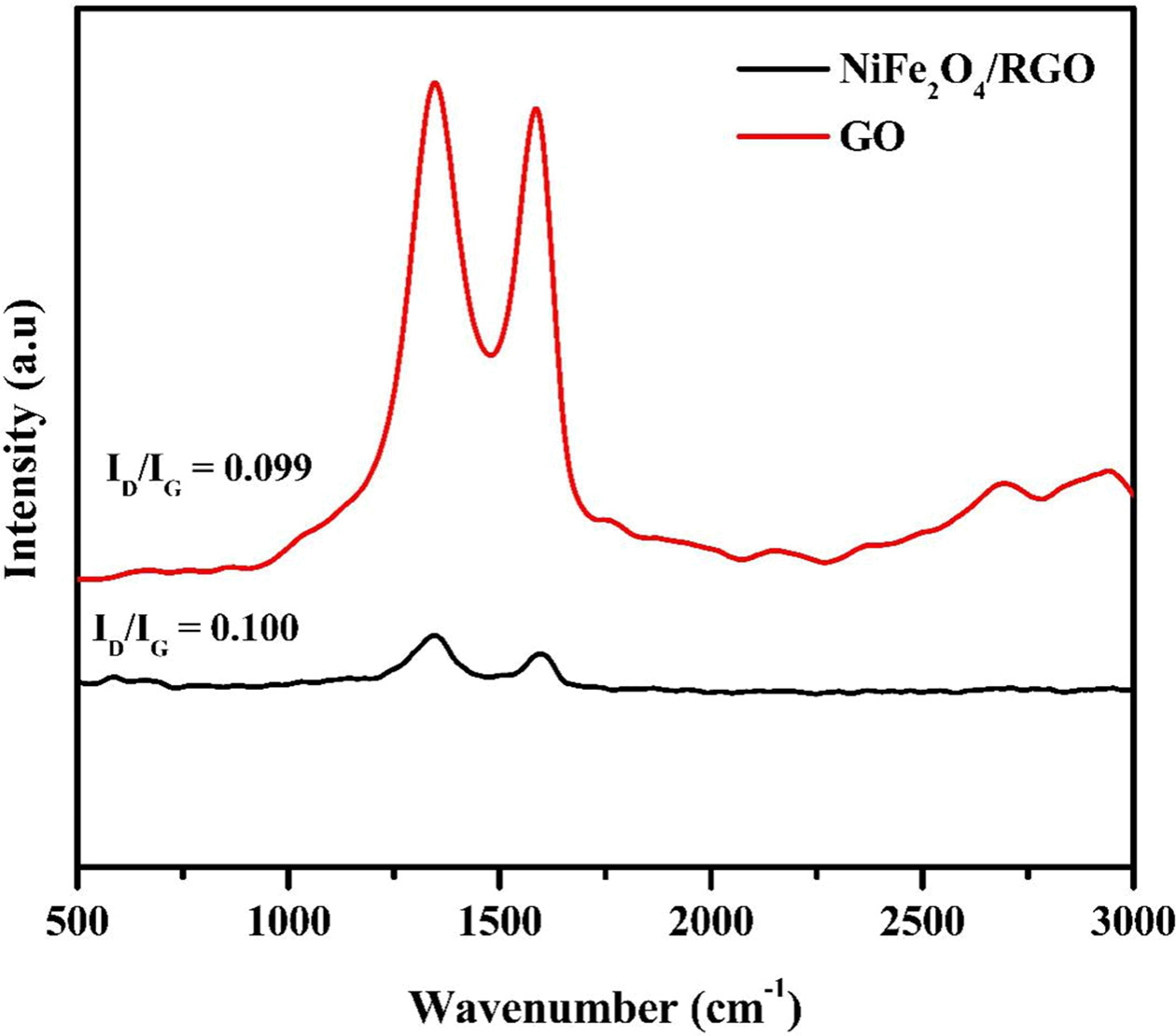
Fig. 3 shows the Raman spectrum of GO
and NiFe2O4/1% RGO. Two prominent peaks at 1,339 cm-1
and 1,589 cm-1
were observed in the Raman spectrum of GO corresponding to D and G band
respectively. The G band corresponds to the E2g mode linked to sp2
carbon vibrations and D band corresponds to that of sp3 carbon atoms of defects
and disorders. In addition to D and G band few other bands with lower intensity
in the spectral range of 500-700 cm-1
were observed for NiFe2O4/rGO which are characteristic bands for
the spinel type NiFe2O4.
The ID/IG value of the rGO composite was higher (1.1)
compared to GO (0.99) which indicates the presence of sp3 defects upon
reduction or interaction with NiFe2O4.
Electrical
characterisation
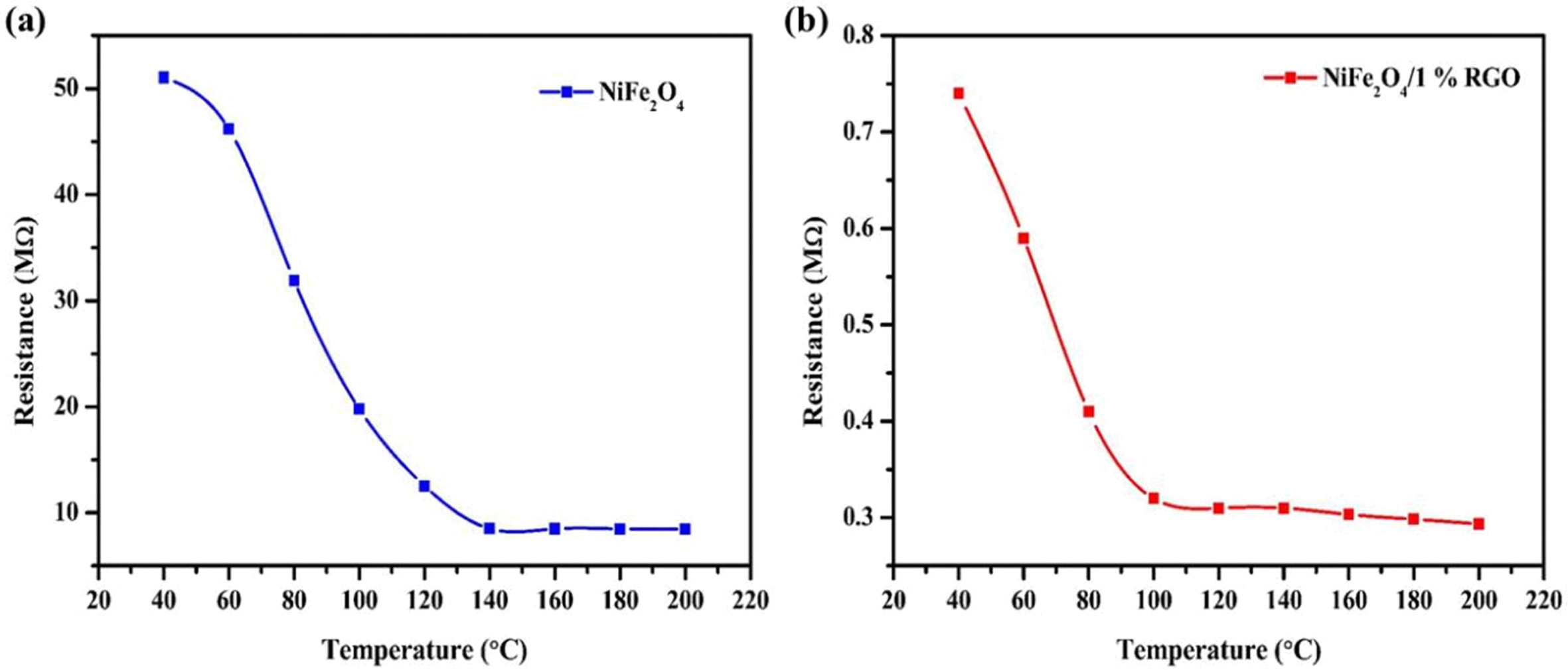
The resistance of NiFe2O4 and NiFe2O4/rGO
with the raise in temperature is measured and is shown in Fig.
4. Since NiFe2O4 being n type in nature exhibited a
reduction in resistance with an increase in temperature. Also due to the combination
of NiFe2O4 with a highly conductive rGO sheets, a
relatively higher resistance decrease is observed. Similar
improvement in conductivity of metal oxides WO3 and
ZnO anchored on rGO sheets were observed by Qin et al and Anand et al.
H2
sensing Characteristics
To determine the optimum temperature for H2
sensing, temperature studies were performed from 40 ºC to 200 ºC
in steps of 20 ºC and at each temperature a constant H2
concentration of 200 ppm was maintained. Fig.
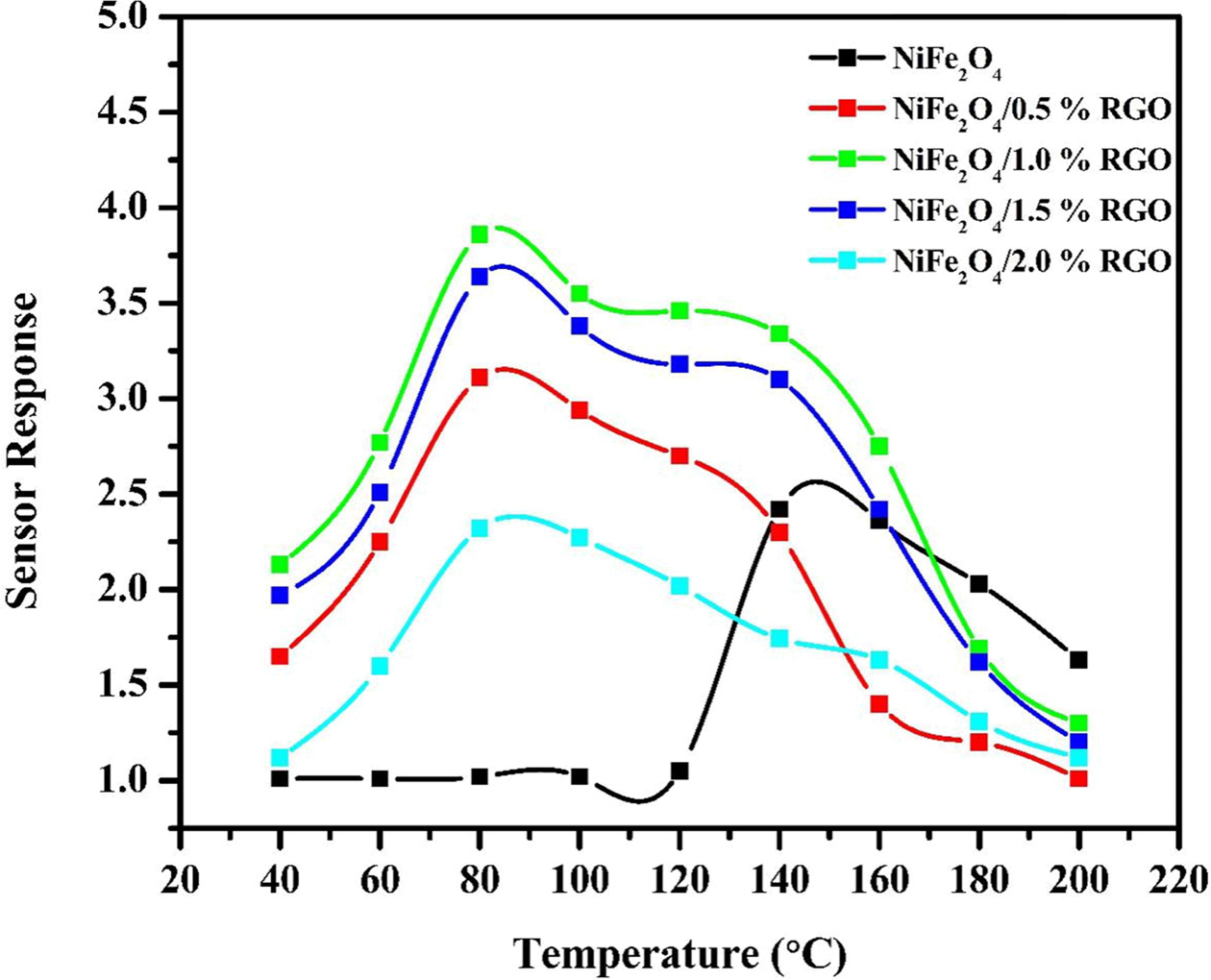
5 shows the sensor response vs. operating temperature of pristine NiFe2O4
and NiFe2O4/rGO nanocomposite at various additions of
rGO. From the Fig it can be clearly observed that with the increase in
temperature the conductivity increases. All the rGO composites
showed the maximum sensor performance at an
optimum operation temperature of 80 ºC. On the other hand, NiFe2O4
showed relatively lower performance at an operating temperature of 140 ºC. With
addition of rGO, there is a clear improvement in the sensing
performance. The enhanced performance is
attributed to the incorporation of NiFe2O4
into rGO which increases the effective active
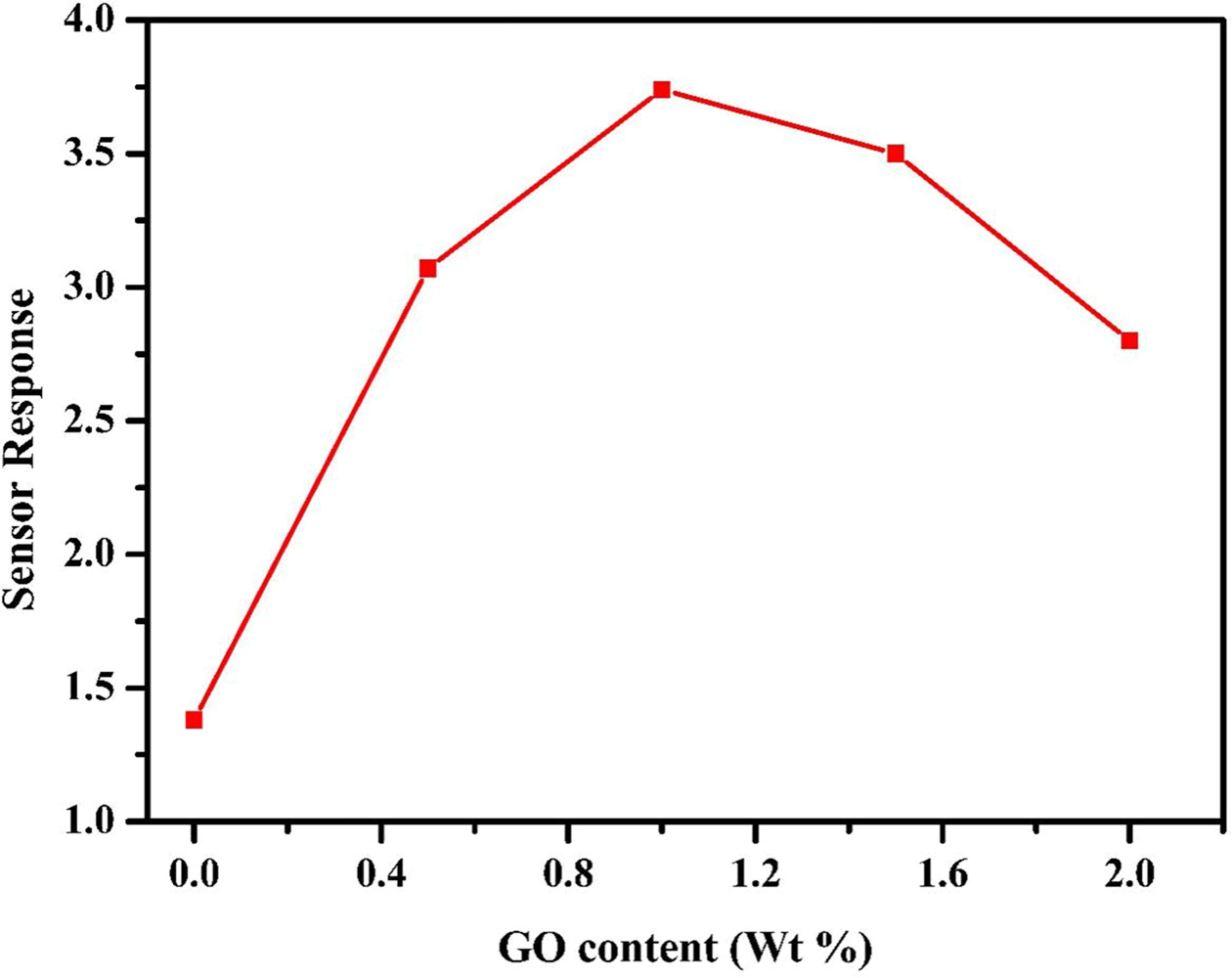
surface area of gas interaction. Also, it can be seen that NiFe2O4/1%
rGO marked the highest performance among all the different
compositions and hence was chosen for subsequent sensing characterization. With
increase in rGO concentration above 1%, there is a possibility
of agglomeration which could lead to decreased active
surface area and hence low performance.
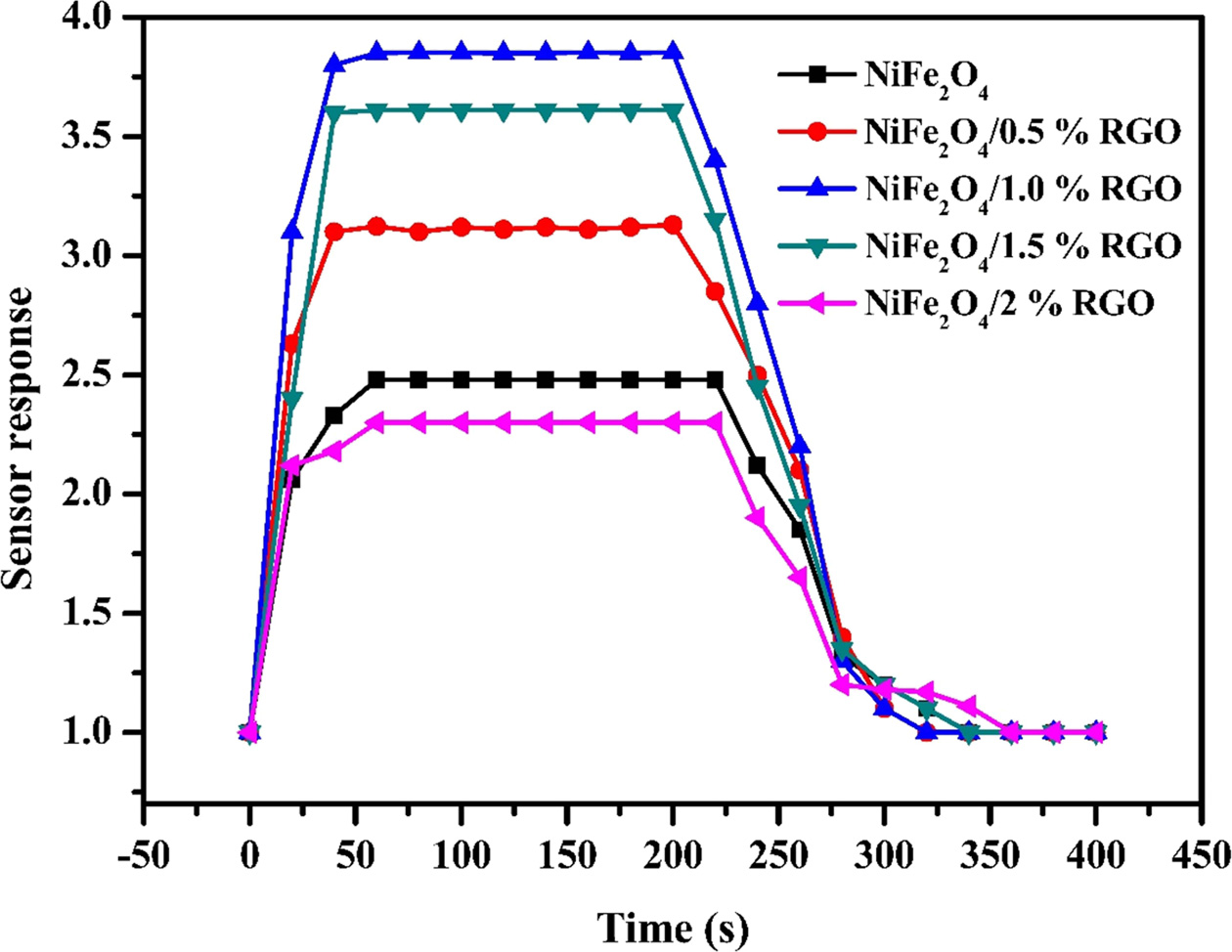
To determine the response and recovery time, pristine NiFe2O4
and NiFe2O4/1% rGO was subjected to 200 ppm of H2
at their operating temperature. Fig. 6 shows
that the composite showed quicker response of 28 s and recovery time of 85 s.
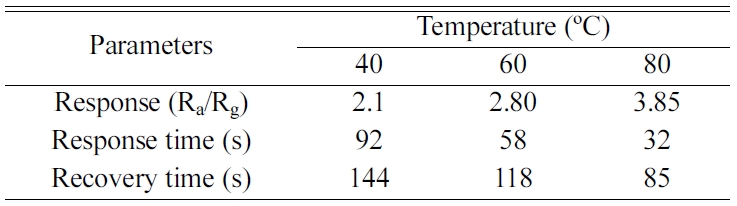
To further comprehend the effect of temperature on sensor
performance, the response time and recovery time were measured
at various operating temperatures for NiFe2O4/1
% rGO composite and the results are given in Table 1. From the
table it can be visualized that with the increase in temperature there is a
reduction in both response and recovery time. Also at
temperature beyond 80 ºC there is no significant change in
the response and recovery times. In fact with higher temperature the peak response
value degraded as observed in Fig. 5.
Detection
of H2 gas
The NiFe2O4/1% rGO composite sensor
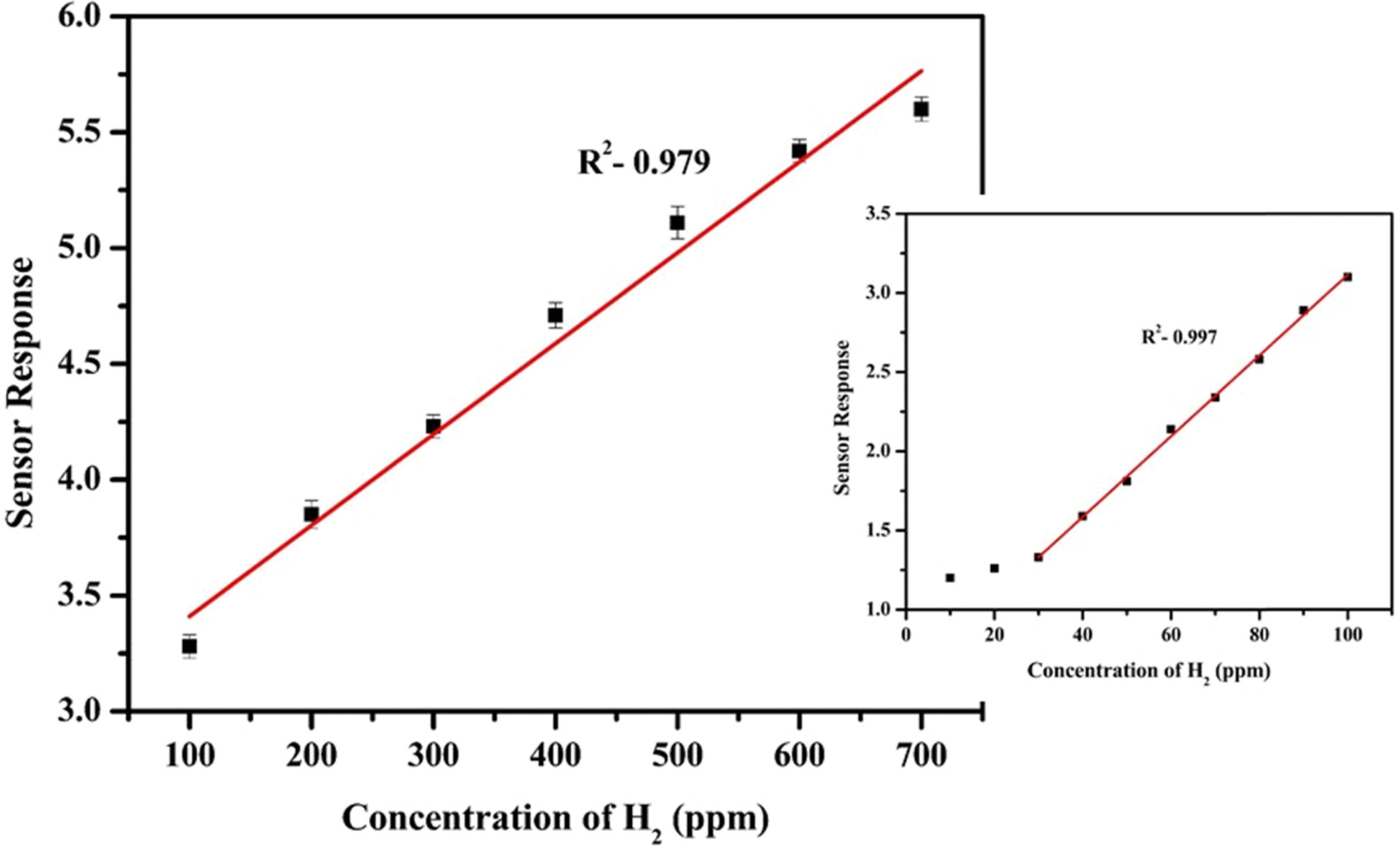
performance towards different concentration of H2 is depicted in Fig.
7. At higher concentration from 100 ppm to 700 ppm, a linear response trend was
observed. Beyond 800 ppm there was no significant rise in the response due to
the saturation of the active adsorption sites. At lower concentrations from 10
ppm to 100 ppm, there was no linearity, however there was a steep linear
response after a concentration of 30 ppm. Such kind of response is
due to the fact that at lower concentrations, the gas molecules
adsorbed to the active sites form a monolayer. With an increasing
concentration, subsequent layers are
formed thereby the response is dominated by the multilayer
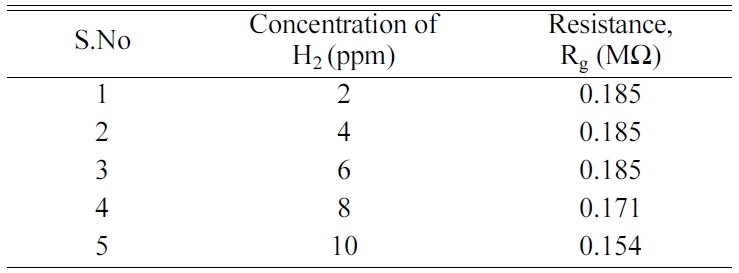
adsorption. The sensor showed a limit of detection
(LOD) of 8 ppm. LOD is the lowest concentration of gas detected
by the sensor. The detection limit was determined by supplying
known volume of gas into the test chamber and measuring the change in
resistance as shown in Table 1. The resistance given by NiFe2O4/1%
rGO in air is 0.185×106 Ω. A significant change in the
resistance for a particular gas concentration was
regarded as the lowest detection limit of the sensor. The H2
sensor characteristics exhibited by NiFe2O4/rGO is
compared with other metal oxides and is shown in Table 2.
Interference
studies
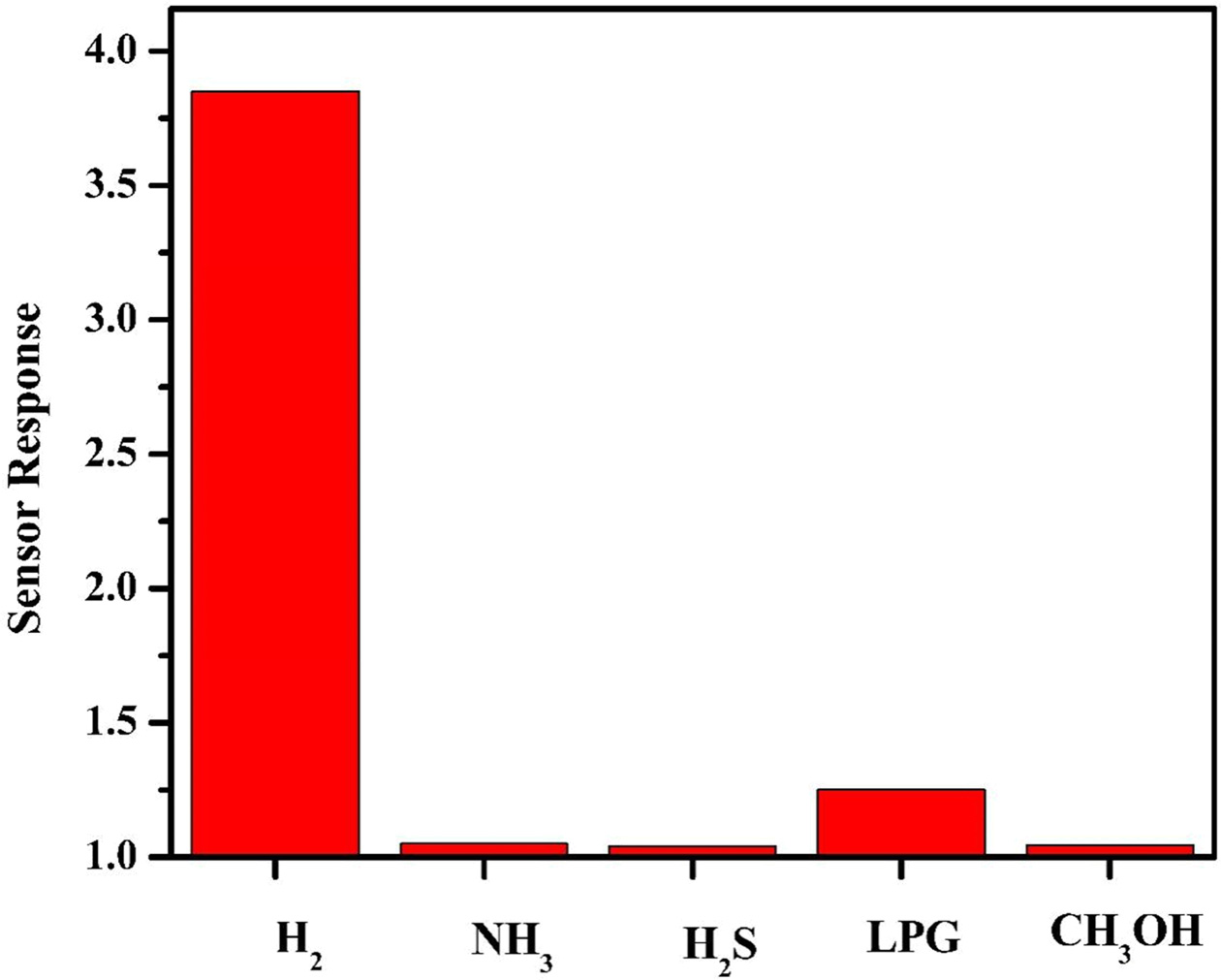
To determine the selectivity of the sensor, it was exposed
to gases such as ammonia, H2S2, ethanol and LPG at 80 ºC.
Fig. 7 shows the interference plot for 200 ppm of H2 and equal
volumes of the other gases. Only around 10% response was exhibited by the
sensor for ammonia, H2S2 and ethanol showing a remarkable
selectivity for H2. However there was a 20% response for LPG leading
to small but significant interference.
Stability
studies
The stability of the sensor was determined by maintaining a concentration of 200 ppm and measuring the resistance continuously for 1 h by continuous hydrogenation and dehydrogenation for every 6 min (Results not shown). Almost 93% of the response was retained after 6 cycles
which shows the excellent reproducibility and stability of the sensor.
Fig. 8 Fig. 9
|
Fig. 1 TEM images of (a) NiFe2O4 (b) NiFe2O4/1% rGO and (c) SAED pattern of NiFe2O4/1 % rGO. |
|
Fig. 2 XRD pattern of NiFe2O4 and NiFe2O4/1% rGO. |
|
Fig. 3 Raman spectrum of NiFe2O4 and NiFe2O4/1% rGO. |
|
Fig. 4 Resistance Vs. temperature response curves of (a) NiFe2O4 and (b) NiFe2O4/1% rGO. |
|
Fig. 5 Sensing response vs. operating temperature of NiFe2O4 and NiFe2O4/rGO for a H2 concentration of 200 ppm. |
|
Fig. 6 Sensor response towards 200 ppm of H2 for different GO content in NiFe2O4/rGO composite at an optimum temperature of 80 ᵒC. |
|
Fig. 7 Sensor response vs. time graph of NiFe2O4 and NiFe2O4/rGO composite for a H2 concentration of 200 ppm at their respective operating temperature. |
|
Fig. 8 Sensing response of NiFe2O4/1% rGO at operating temperature for H2 concentration from 100 to 700 ppm and inset shows sensor response for 10-100 rpm. |
|
Fig. 9 Interference plot of NiFe2O4/1% rGO showing sensor response for 200 ppm of different gases at an operating temperature of 80 ᵒC. |
|
Table 1 NiFe2O4/1% rGO sensor response and reaction kinetics vs operating temperature |
|
Table 2 Resistance values of NiFe2O4/1% rGO for H2 concentration from 2 ppm-10 ppm |
NiFe2O4/rGO were prepared by a
facile hydrothermal method and employed as a chemiresistive H2
sensor. The synthesized materials were characterized by TEM, XRD and Raman
spectroscopy which showed the morphology and structural pattern of the
composite. The electrical characterization showed the n type behavior of both
pristine NiFe2O4 and NiFe2O4/rGO thereby with the adsorption of gas increase in
conductivity is expected to occur.
The gas sensing characteristics of the composite were studied at different
operating temperature with four variants. The nanocomposite with 1 wt % rGO showed a higher response to H2 sensing at
an operating temperature of 80 ºC. Also a very low response and recovery time
of 32 s and 85 s were observed. Further the sensor showed good sensitivity,
selectivity and stability in the presence of interfering gases. It is concluded
that with the incorporation
of rGO there is an increased electron transfer facilitating a decrease in
resistance and thereby better performance.
- 1. T. Hübert, L. Boon-Brett, G. Black, and U. Banach, Sensor Actuat. B Chem. 157 (2011) 329-352.
-

- 2. V. Tozzini, and V. Pellegrini, Phys. Chem. Chem. Phys. 15 (2013) 80-89.
-

- 3. P. Jena, J. Phys. Chem. Lett. 2 (2011) 206-211.
-

- 4. L. Schlapbach, and A. Züttel, in Materials for sustainable energy a collection of peer-reviewed research and review articles from nature publishing group. (2011) 265-270.
-

- 5. L. Boon-Brett, J.Bousek, and P.Moretto, Int. J. Hydrog. Energy 34 (2009) 562-571.
-

- 6. F. Favier, E.C. Walter, M.P. Zach, and T. Benter, and R.M. Penner, Science 2001293 (5538) 2227-2231.
-

- 7. S. Dhall, K. Sood, and R. Nathawat, Int. J. Hydrog. Energy 42 (2017) 8392-8398.
-

- 8. A. Kaniyoor and S. Ramaprabhu, Carbon 49 (2011) 227-236.
-

- 9. F.L. Meng, Z. Guo, and X.J. Huang TRAC-Trends Anal. Chem. 68 (2015) 37-47.
-

- 10. N. Joshi, T. Hayasaka, Y. Liu, H. Liu, O.N. Oliveira, and L. Lin Microchim.Acta 185 (2018) 213.
-

- 11. T. Wang, D. Huang, Z. Yang, S. Xu, G. He, X. Li, N. Hu, G. Yin, D. He, and L. Zhang, Nano-Micro Lett. 8 (2016) 95-119.
-

- 12. F.L. Meng, Z. Guo, and X.J. Huang TRAC-Trends Anal. Chem. 68 (2015) 37-47.
-

- 13. K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, and A. Firsov, Science 306 (2004) 666-669.
-

- 14. S.S. Varghese, S. Lonkar, K.K. Singh, S. Swaminathan, and A. Abdala, Sensor Actuat. B Chem. 218 (2015) 160-183.
-

- 15. N. Joshi, T. Hayasaka, Y. Liu, H. Liu, O.N. Oliveira, and L. Lin, Mikrochim.Acta 185 (2018) 213.
-

- 16. A. Pandey, N.R. Wilson, and J.A. Covington, Sensor Actuat. B Chem. 183 (2013) 478-487.
-

- 17. M.G. Chung, D.H. Kim, D.K. Seo, T. Kim, H.U. Im, H.M. Lee, J.B. Yoo, S.H. Hong, T.J. Kang, and Y.H. Kim, Sensor Actuat. B Chem. 169 (2012) 387-392
-

- 18. B. Alfano, T. Polichetti, M.L. Miglietta, E. Massera, C. Schiattarella, F. Ricciardella, and G. Di Francia, Sensor Actuat. B Chem. 239 (2017) 1144-1152.
-

- 19. D. Gupta, D. Dutta, M. Kumar, B. Barman, C.K. Sarkar, S. Basu, and S.K. Hazra, Sensor Actuat. B Chem. 196 (2014) 215-222.
-

- 20. J.X. Wang, X.W. Sun, Y. Yang, H. Huang, Y. Lee. O.K. CTan, and L. Vayssieres, Nanotechnology 17 (2006) 4995.
-

- 21. W. Wu, M Zeng, and Y. Li, Mater.Lett. 104 (2013) 34-36.
-

- 22. M.A Kozhushner, L.I. Trakhtenberg, A.C. Landerville, and I.I. Oleynik, J. Phys. Chem. C 117 (2013) 11562-11568.
-

- 23. S.L. Darshane, S.S. Suryavanshi, and I.S. Mulla, Ceram. Int. 35 (2009) 1793-1797.
-

- 24. H.G. Na, Y.J. Kwon, S.Y. Kang, W. Kang, M.S. Choi, J.H. Bang, T.K. Jung, C. Lee, and H.W. Kim, J Ceramic Processing Research 17[6] (2016) 523-531.
- 25. S. Rathore, D.K. Patel, and P.-D. Hong, J Ceramic Processing Research 20[4] (2019) 442-448.
- 26. G. Korotcenkov, Sens. Actuators B 107 (2005) 209-232.
-

- 27. R. Hajihashemi, A.M. Rashidi, M. Alaie, R. Mohammadzadeh, and N. Izadi, Mater. Sci. Eng. C 44 (2014) 417-421.
-

- 28. J.Y. Patil, D.Y. Nadargi, J.L. Gurav, I.S. Mulla, and S.S. Suryavanshi, Mater. Lett. 124 (2014) 144-147.
-

- 29. R.B. Kamble and V.L. Mathe, Sensor Actuat. B Chem. 131 (2008) 205-209.
-

- 30. Y. Fu, Y. Wan, H. Xia, and X. Wang, J. Power Sources 213 (2012) 338-342.
-

- 31. Y. Xiong Fu, L. Wang, and X. Wang, Chem. Eng. 195 (2012) 149-157.
-

- 32. W. Zhang, M.B. Jungfleisch, W. Jiang, J.E. Pearson, and A. Hoffmann, J. Appl. Phys. 117 (2015) 17C727.
-

- 33. A. Mukherjee, S. Chakrabarty, W.N. Su, and S. Basu, Mater.Today Energy 8 (2018) 118-124.
-

- 34. F. Liu, X. Chu, Y. Dong, W. Zhang, W. Sun, and L. Shen, Sensor Actuat. B Chem. 188 (2013) 469-474.
-

- 35. Q. Lin, Y. Li, and M. Yang, Sens. Actuators B 173 (2012) 139-147.
-

- 36. W.S. Hummers, R.E. Offeman, J. Am. Chem. Soc. 80 (1958) 1339.
-

- 37. J. Qin, M. Cao, N. Li, and C. Hu, J. Mater. Chem. 21 (2011) 17167-17174.
-

- 38. K. Anand, O. Singh, M. Singh, J. Kaur, and R.C. Singh, Sensor Actuat. B Chem. 195 (2014) 409-415.
-

- 39. A. Russo, N. Donato, S.G. Leonardi, S. Baek, D.E. Conte, G. Neri, and N. Pinna Angew. Chem. Int. Ed. 51 (2012) 11053-11057.
-

- 40. A. Venkatesan, S. Rathi, I.Y. Lee, J. Park, D. Lim, G.H. Kim, and E.S. Kannan, Semicond. Sci. Technol. 31 (2016) 125014.
-

- 41. A. Pandey, N.R. Wilson, and J.A. Covington, Sensor Actuat. B Chem. 183 (2013) 478-487.
-

- 42. F. Bayata, B. Saruhan-Brings, and M. Ürgen, Sensor Actuat. B Chem. 204 (2014) 109-118.
-

- 43. A. Katoch, Z.U. Abideen, H.W. Kim, and S.S. Kim, ACS Appl. Mater & Interfaces 8 (2016) 2486-2494.
-

- 44. C. Xiang, Z. She, Y. Zou, J. Cheng, H. Chu, S. Qiu, H. Zhang, L. Sun, and F. Xu, Ceram. Int. 40 (2014) 16343-16348.
-

- 45. L. Huo, X. Yang, Z. Liu, X. Tian, T. Qi, X. Wang, K. Yu, J. Sun, and M. Fan, Sensor Actuat. B Chem. 244 (2017) 694-700.
-

- 46. K. Nguyen, C.M. Hung, T.M. Ngoc, D.T.T. Le, D.H. Nguyen, D.N. Van, and H.N. Van, Sensor Actuat. B Chem. 253 (2017) 156-163.
-

 This Article
This Article
-
2020; 21(3): 343-350
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.343
- Received on Mar 12, 2020
- Revised on Mar 20, 2020
- Accepted on Mar 20, 2020
 Services
Services
Shared
 Correspondence to
Correspondence to
- J. Niresh
-
Assistant Professor, Department of Automobile Engineering, PSG College of Technology, Coimbatore, India
Tel : +91422 4344281 - E-mail: nireshcbe@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.