- Effects of V2O5 on sinterability and microwave dielectric properties of NaCa4V5O17 ceramics
Guoguang Yao*, Yang Li, Jingjing Tan, Cuijin Pei, Yan Zhang and Jia Chen
School of Science, Xi’an University of Posts and Telecommunications, Xi’an 710121, China
The NaCa4V5O17
ceramics owing low sintering temperature had been prepared via conventional
solid state reaction method using V2O5 as vanadium
source. The sinterability, microwave dielectric characterisations and
compatibility with Ag were investigated. Pure phase NaCa4V5O17
with triclinic structure was confirmed by Rietveld refinement and Raman
spectrum. The permittivity (εr) and quality factor (Qxf)
values mainly depended on the relative density, whereas the temperature
coefficient of resonant frequency (τf) value was closely connected
with the tetrahedral distortion of V(1)O4. The NaCa4V5O17
ceramics sintered at 800 oС owned high densification and moderate microwave
dielectric performances under 10.7 GHz: εr = 9.5, Q×f = 34,200 GHz, τf
= -90.0 ppm/oС, but poor chemical compatibility with Ag paste.
Keywords: Ceramics, microwave dielectric properties, NaCa4V5O17, tetrahedral distortion
In the future 10 years, 5G wireless communications will
become the dominant wireless protocol for applications
like Artificial Intelligence and the Internet of Things [1].
The rapid evolution of 5G wireless communications gives rise to higher
requirement of microwave dielectric ceramics [2]. To address the requirements
of 5G wireless communications, the dielectric ceramics must satisfy following primary
dielectric characteristics: low εr (εr ≤ 10) to increase
signal transmission velocity, near-zero τf to ensure thermal stabilization at different operating temperatures, and
high Qxf or low dielectric loss to decrease the power dissipation [3, 4].
Meanwhile, low sintering temperatures should be ensured to meet low-temperature co-sintered ceramic technology, which
can be used to reduce the size of
electronic devices [5, 6]. Therefore, the dielectric materials
with superior performances and inherent lower sintering temperature received prodigious attention.
Recently, vanadium host compounds are followed with
interest once again since their low sintering temperature
and superior dielectric performances. Lots of vanadium-basic ceramics have been
investigated for LTCC substrate applications, for example Ca5Co4(VO4)6,
(CaBi)(MoV)O4, LiMgVO4, (Bi, Ce)VO4, etc
[7-11]. Quite recently, a novel NaCa4V5O17
compounds with triclinic structure had been fabricated by Xie et al. [12].
Later, Fang et al. [13] first presented the microwave dielectric performances
(er = 9.72, Q×f = 51,000 GHz, τf = -84 ppm/oС)
of NaCa4V5O17 ceramics. Considering the
microwave dielectric performances of the ceramics are strongly
depended on the craft parameters, including primary materials,
ball milling and sintering conditions etc. [14]. The variation in one or more
these parameters greatly influence the dielectric properties of such ceramics
[15]. However, there is no related microwave dielectric properties study about
NaCa4V5O17 ceramics using V2O5
as vanadium source. Here, we reported the effects V2O5,
as the vanadium source, on sinterability, microstructure, microwave dielectric
performances as well as compatibility with silver of NaCa4V5O17
ceramics.
We
fabricated NaCa4V5O17 samples through the
traditional solid-state route. V2O5 (99%), Na2CO3
(99.8%) and CaCO3 (99%) were weighed
according to stoichiometric NaCa4V5O17, and
then were ball milled for 8 h using alcohol and ZrO2 balls for
grinding media. The resultant milled powders were dried under 80 oC,
followed by presintered under 600 oC/4 h, and then regrinded for
further 8 h. The above presintered powders were mixed with 5wt % PVA,
granulated and sieved through a No. 80 sieve. The granules were pressed at 200
MPa into compacts (Ф10 mm×5 mm). These compacts were heated at 500 oC
for 2 h to exclude PVA, and then fired in air ambient under 750-850 oС
dwelling for 4 h.
The phase constitutions in sintered specimens had been
studied by X-ray powder diffraction (XRD, Smartlab, Japan) and
Raman spectra (Jobin Yvon, Longjumeau, France) equipped with He-Ne laser and an
output of 30 mW. Rietveld refinement of XRD patterns was carried out with the GSAS
program [16]. Microstructures of NaCa4V5O17
ceramics were observed through a scanning
electron microscope (SEM, Hitachi, Tokyo, Japan). Archimedes' principle was
carried out to determine the bulk densities. The εr and Qxf values
of samples were evaluated by resonant cavity method using Rohde & Schwarz
ZVB20 vector network analyzer under about 10-12 GHz. The τf value of
samples was calculated between 25 and 85 °C according to Reference
[17].
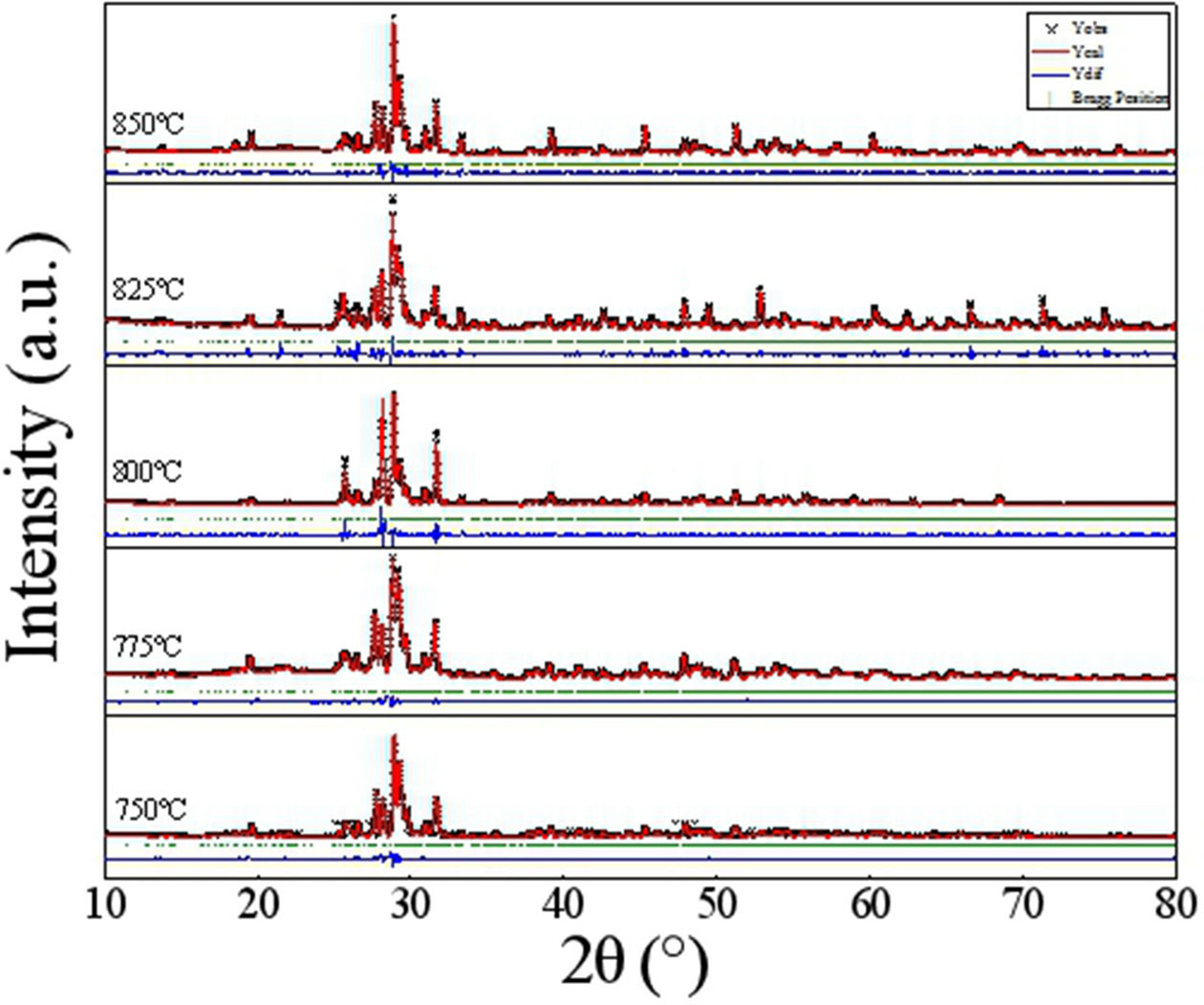
Fig. 1 exhibits the refined fit of NaCa4V5O17
sintered under diverse temperatures to their diffraction patterns. The
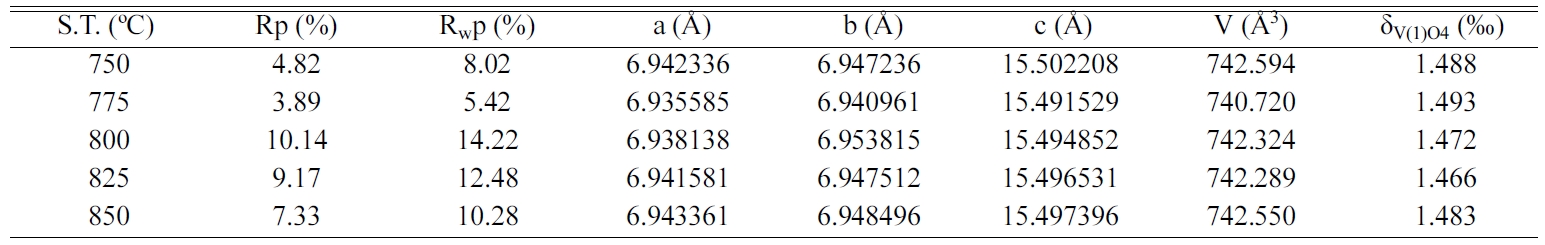
Rietveld refined lattice parameters, reliability factors
and V(1)O4 tetrahedral distortion for all specimens are enumerated
in Table 1. As shown in Fig. 1, the calculated XRD profiles
based on NaCa4V5O17 structural model closely
fitted those of experimental ones, indicating the NaCa4V5O17
ceramics crystallized in a triclinic structure with P-1(2)
space group. Pure phase NaCa4V5O17 without
an obvious secondary phase was obtained for all samples
sintered in the range of 750-850 oC. Moreover, as seen in Table
1, the achieved reliability factors (Rp, Rwp) are less than 15, suggesting the
refined results are credible. Meanwhile, no obvious changes of unit cell volume
were observed under different sintering temperatures.
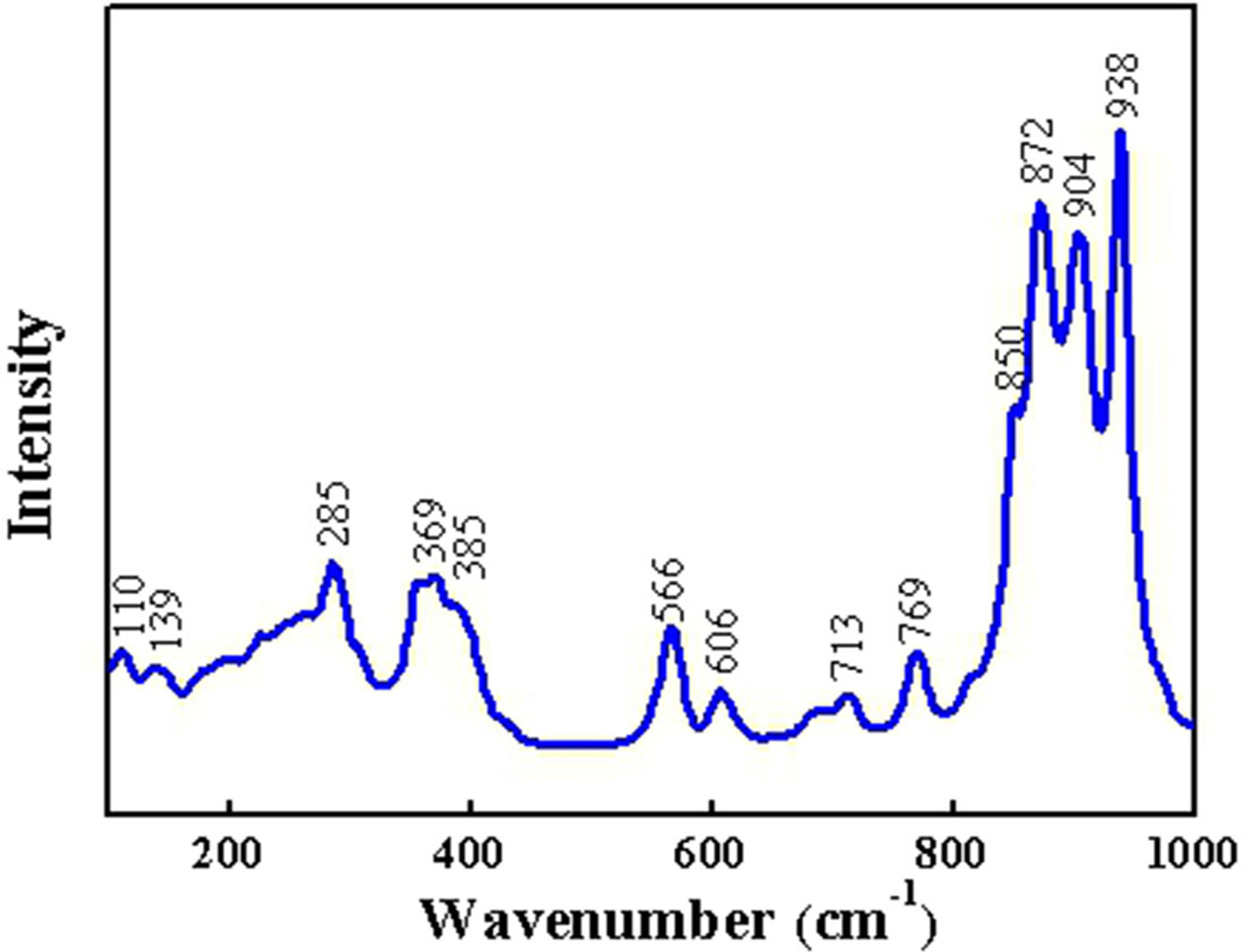
The Raman spectrum of NaCa4V5O17
sample fired under 800 oC is displayed in Fig. 2. Three
distinct regions of Raman modes can be distinguished. The first region located
at 900-940 cm-1
is associated with terminal V-O (V = O) symmetric stretching vibrations [12].
The second region located at 550-880 cm-1
is related to the V-O-V antisymmetric or symmetric stretching vibrations [18].
The third region below the 400 cm-1
is ascribed to the peripheral modes [19].
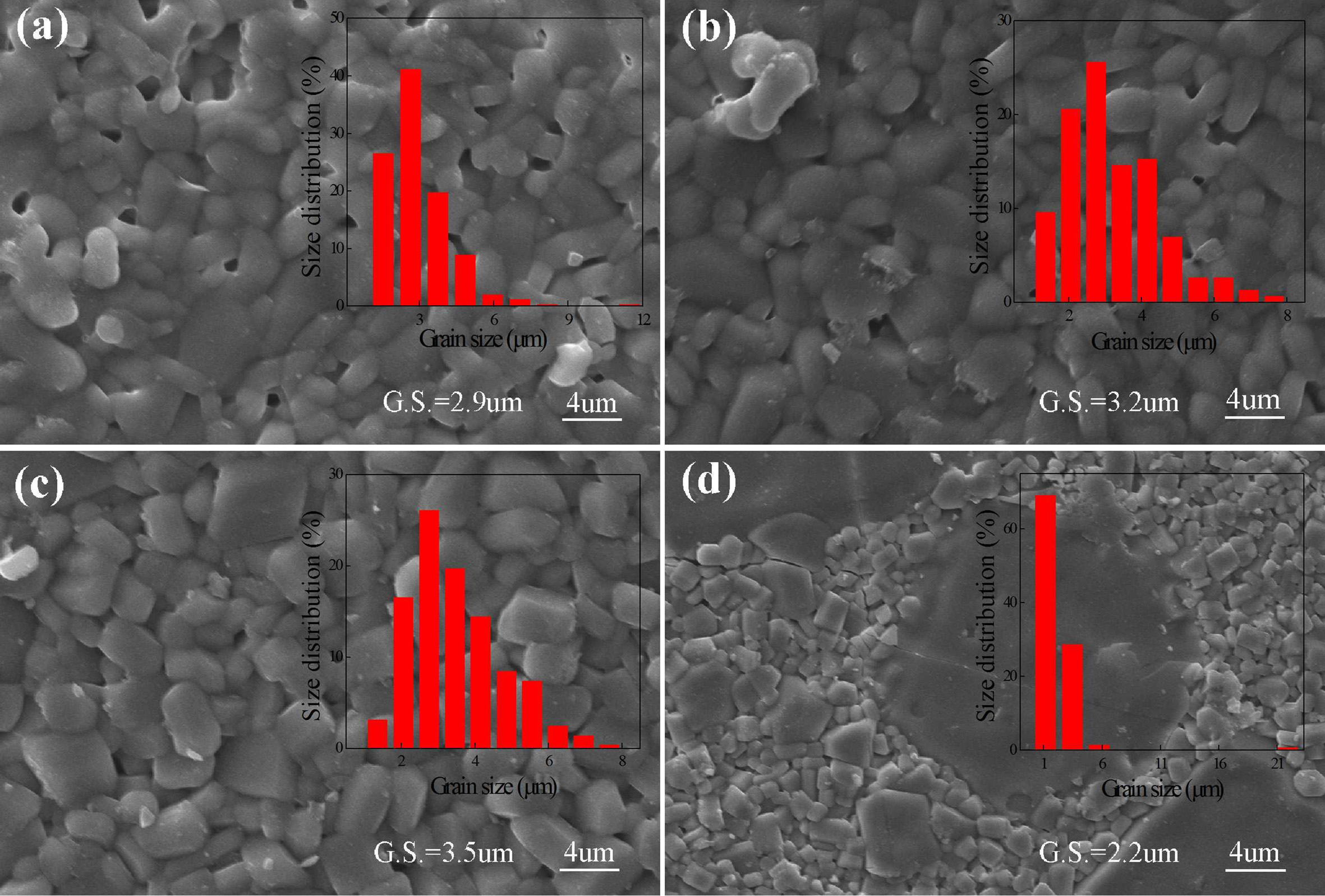
Fig. 3 gives the typical SEM micrographs of NaCa4V5O17
ceramics heated at distinct temperatures. From Figs. 3(a)-(c), the number of
residual open pores was reduced accompanied by average grain size growth with
an elevated temperature. And the 800 oC-sintered sample
presented a relative compact and uniform microstructure with a mean grain size
around 3.5 um, which is helpful to improve the dielectric properties of the
NaCa4V5O17 ceramics [20]. When the sintering
temperature rose to 825 oC, excessive grain growth, uneven
grain distribution and crack formed, these would deteriorate the dielectric
performances of the present ceramics [21].
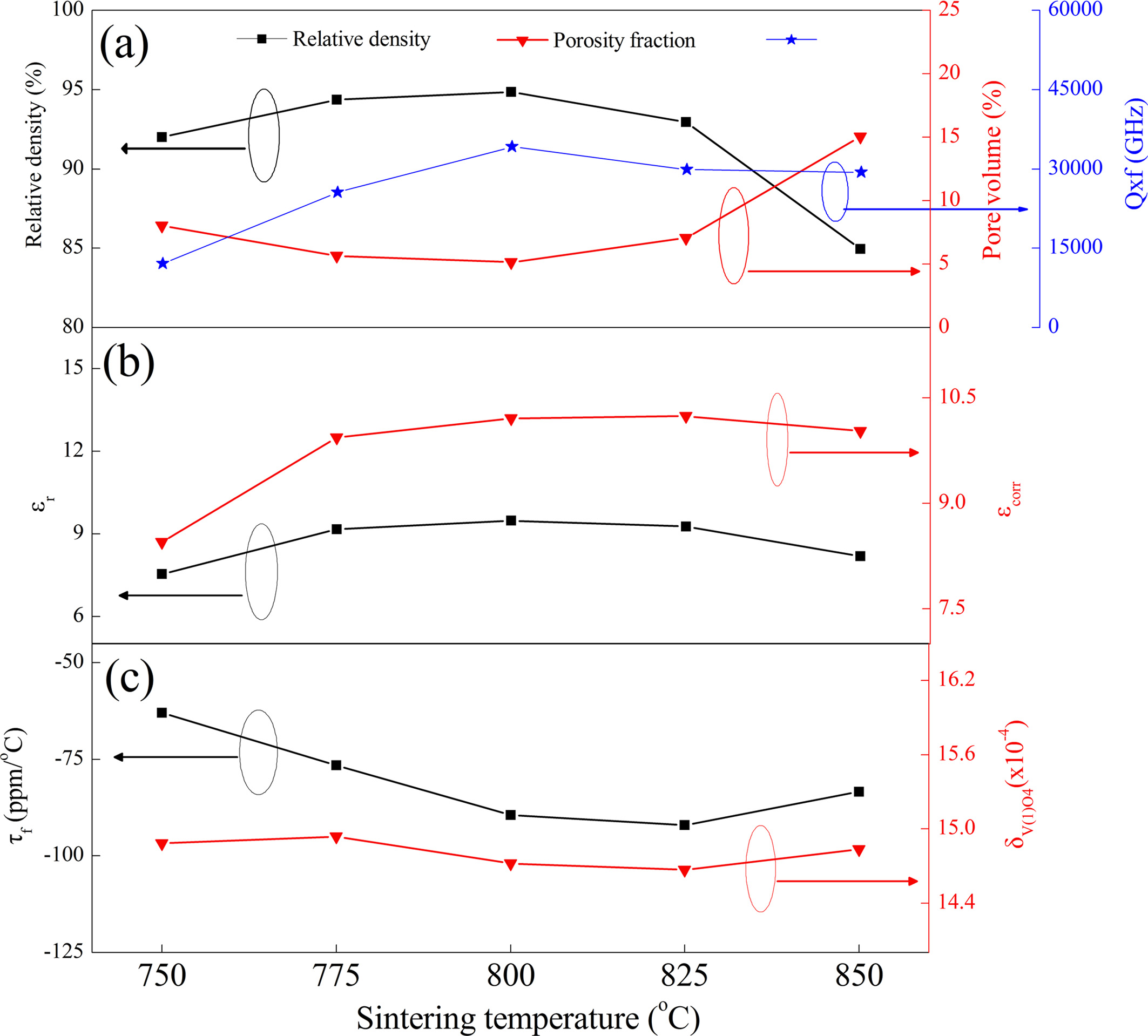
Fig. 4 presents the variations in relative density, pore volume,
microwave dielectric properties and tetrahedral distortion
of NaCa4V5O17 ceramics heated at 750-850 oC.
As shown in Fig. 4(a), the relative density was enhanced to the maximum (94.9%)
under 800 oC and thereafter declined with an elevated
temperature, which was agree with the above morphology analysis. In general,
the Qxf value at microwave region of dielectric ceramics is
predominated by extrinsic parameters like relative density, second phase and
oxygen vacancies [22-24]. Considering the similar variation tendency between
Qxf value and relative density of NaCa4V5O17
ceramics, suggesting the Q×f value was predominated by the relative density.
The εr is determined by density, dielectric polarizability and
molecular volume [25, 26]. For a given compounds, its εr mainly
depends on the relative density or porosity owing to the invariant dielectric
polarizabilities and chemical formula [26]. Therefore, the changes of εr
and εcorr of NaCa4V5O17 ceramics
exhibited a tendency inverse or similar to the variation in
porosity or relative density, and the corrected dielectric
constant (εcorr) was calculated according to reference [27]. It was
reported that the τf value could be determined by the distortion of oxygen
polyhedral and phase composition [28, 29], which was also well represented
in the present work. As seen in Fig. 4(c), the change of τf
of NaCa4V5O17 ceramics exhibited a tendency
similar to that of the variation of V(1)O4 tetrahedral
distortion (δV(1)O4), indicating the τf is
greatly affected by δV(1)O4. The δV(1)O4 calculated
based the Rietveld refinement data and Shannon equation [30]. It is
noted that the NaCa4V5O17 ceramics in our
cases possessed compatible εr (9.5) and τf
(-90.0 ppm/oC) but inferior Qxf (34,200 GHz) value than
that of NaCa4V5O17 (εr = 9.72, τf
= -84 ppm/oC, Qxf = 51,000 GHz) reported by Yin et al. [13], which
may be due to the different vanadium source and processing
conditions [31].
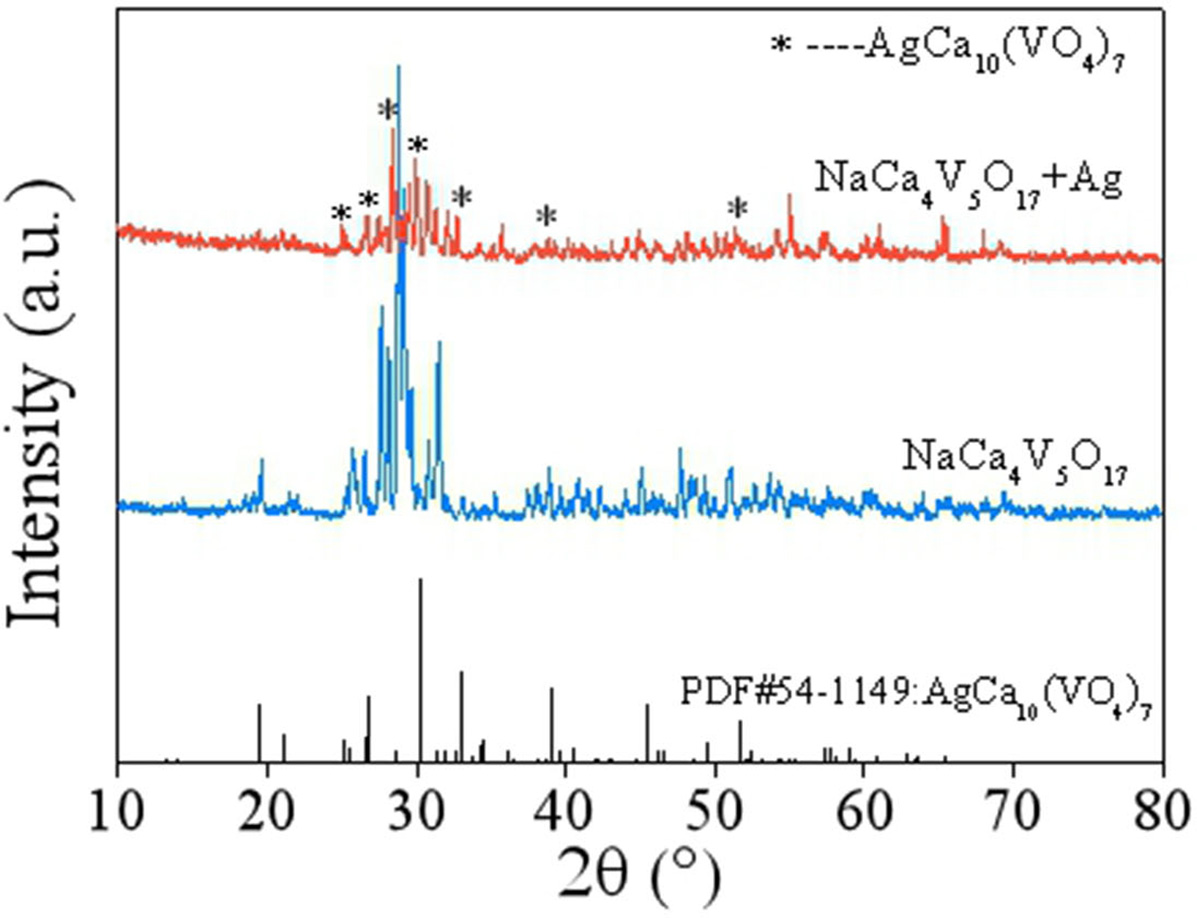
Fig. 5 depicts the XRD patterns of NaCa4V5O17
presintered powder with 20 wt% Ag powder co-fired under 800 oC. As
seen from Fig. 5, a new main phase AgCa10(VO4)7
(JCPDS#54-1149) formed except for NaCa4V5O17
phase, which was also not agree with the previous
report [13]. This result showed that a chemical reaction
between the basic phase NaCa4V5O17 and Ag
happened, which impeded its further application for LTCC.
|
Fig. 1 Rietveld refinement patterns of NaCa4V5O17 ceramics sintered at deferent temperatures. |
|
Fig. 2 Raman spectrum of NaCa4V5O17 ceramics sintered at 800 oC. |
|
Fig. 3 SEM micrographs of NaCa4V5O17 ceramics sintered at (a) 750 oС, (b) 775 oС, (c) 800 oС, (d) 825 oС. |
|
Fig. 4 Dependence of ρr, pore volume, εr, Qxf, τf and δV(1)O4 on the firing temperature of NaCa4V5O17 ceramics. |
|
Fig. 5 XRD patterns of NaCa4V5O17-20 wt% Ag mixture co-fired at 800 oC. |
|
Table 1 Rietveld refinement results and V(1)O4 tetrahedral distortion (δV(1)O4) of NaCa4V5O17 ceramics sintered at various temperatures. |
The NaCa4V5O17 ceramics
owing low firing temperature had been prepared via the route of
conventional solid state reaction. The impacts of V2O5 on
the sinterability, microwave dielectric characterizations as well as chemical compatibility
with Ag of present ceramics were
investigated. The XRD and Raman spectrum revealed that the NaCa4V5O17
ceramics crystallized in a triclinic structure with P-1(2) space group. The εr
and Qxf values were dominated by the relative density, whereas the τf
value was closely connected with the tetrahedral distortion of V(1)O4.
Typically, the 800 oС-sintered
NaCa4V5O17 ceramics owned moderate microwave dielectric performances at measured frequency of
10.7 GHz: er = 9.5, Q×f = 34,200 GHz, τf = -90.0 ppm/oС,
whereas its poor chemical compatibility with Ag paste affected its practical viability
for LTCC applications.
This study was financially supported by China Post-doctoral Science Foundation
(2015M582696), Shaanxi Province Postdoctoral Science Foundation, Education
Department of Shaan xi Province (18JK0711), Science and technology plan project
of Xi'an Bureau of Science and technology (GXYD17.19), and Innovation Funds of
Graduate Programs of Xi'an Post and Telecom-munications University (Grant Nos.
CXJJLA2018008, CXJJLD2019020).
- 1. Q.B. Lin, K.X. Song, and B. Liu, Ceram. Int. 46 (2020) 1171-1177.
-

- 2. H. Li, C.Y. Cai, Q.Y. Xiang, B. Tang, J. Xiao, and S.R. Zhang, Ceram. Int. 45 (2019) 23157-23163.
-

- 3. J.J. Bian, X.Q. Sun, and Y.R. Xie, J. Eur. Ceram. Soc. 39 (2019) 4139-4143.
-

- 4. W.B. Hong, L. Li, H. Yan, and X.M. Chen, J. Am. Ceram. Soc. 102 (2019) 5934-5940.
-

- 5. X.H. Ma, S.H. Kweon, S. Nahm, C.Y. Kang, and Y.S. Kim, J. Eur. Ceram. Soc. 37 (2017) 605-610.
-

- 6. A. Pirvaram, E. Taheri-Nassaj, W.Z. Lu, and W. Lei, J. Am. Ceram. Soc. 102 (2019) 5213-5222.
-

- 7. R. Naveenraj, E.K. Suresh, and J. Dhanya, Eur. J. Inorg. Chem. 2019 (2019) 949-955.
-

- 8. H.H. Guo, D. Zhou, L.X. Pang, and Z.M. Qi, J. Eur. Ceram. Soc. 39 (2019) 2365-2373.
-

- 9. G.G. Yao, P. Liu, X.G. Zhao, J.P. Zhou, and H.W. Zhang, J. Eur. Ceram. Soc. 34 (2014) 2983-2987.
-

- 10. H.H. Guo, D. Zhou, and W.F. Liu, J. Am. Ceram. Soc. 103 (2019) 423-431.
-

- 11. G.G. Yao, X.S. Hu, P. Liu, and J.G. Xu, J. Mater. Sci: Mater. Electron. 26 (2015) 1795-1798.
-

- 12. Z.Q. Xie, S.C. Cheng, S.W. Li, and H.Q. Ding, J. Solid. State. Chem. 269 (2019) 94-99.
-

- 13. C.Z. Yin, C.C. Li, L. Fang et al., J. Eur. Ceram. Soc. 40 (2020) 386-390.
-

- 14. Y.C. Liou, S.L. Yang, and S.Y. Chu, J. Alloy. Compd. 576 (2013) 161-169.
-

- 15. C.J. Pei, C.D. Hou, Y. Li, G.G. Yao, P. Liu, and H.W. Zhang, J. Alloy. Compd. 792 (2019) 46-49.
-

- 16. A.C. Larson and R.B. Von Dreele, Los Alamos National Laboratory Report LAUR 86 (2004)
-

- 17. G.G. Yao, C.D. Hou, C.J. Pei, and P. Liu, Ferroelectrics. 536 (2018) 156-161.
-

- 18. A. Sharma, M. Varshney, and K.H. Chae, Rsc. Adv. 8 (2018) 26423-26431.
-

- 19. E.K. Suresh, K. Prasad, N.S. Arun, and R. Ratheesh, J. Electron. Mater. 45 (2016) 2996-3002.
-

- 20. S.P. Wu, D.F. Chen, C. Jiang, Y.X. Mei, and Q. Ma, Mater. Lett. 91 (2013) 239-241.
-

- 21. J.B. Song, K.X. Song, J.S. Wei, H.X. Lin, and J.M. Xu, J. Alloy. Compd. 731 (2018) 264-270.
-

- 22. J.M. Kim, H.W. Jo, and E.S. Kim, Int. J. Appl. Ceram. Technol. 16 (2019) 2053-2059.
-

- 23. R.C. Pullar, S.J. Penn, I.M. Reaney, and N.M. Alford, J. Eur. Ceram. Soc. 29 (2009) 419-424.
-

- 24. S.B An, J. Jiang, J.Z. Wang, L. Gan, and T.J. Zhang, Ceram. Int. 46 (2020) 3960-3967.
-

- 25. C.J. Pei, G.G. Yao, and Z.Y. Ren, J. Ceram. Process. Res. 17 (2016) 681-684.
-

- 26. Y. Wang L.W. Shi, and W.S. Xia, Ceram. Int. 46 (2020) 6984-6986.
-

- 27. T. Hanai and Z. Kolloid, Kolloid-Zeitschrift. 171 (1960) 23-30.
-

- 28. K.M. Manu, K.A. Lazar, R. Ubic, and M.T. Sebastian, J. Am. Ceram. Soc. 96 (2013) 1504-1511.
-

- 29. G.G. Yao, J. Ceram. Process. Res. 16 (2015) 4144.
- 30. W.H. Baur, Acta. Crystallogr. Sect. B. 30 (1974) 1195-1215.
-

- 31. M. Li, A. Feteira, M.T. Lanagan, and C.A. Randall, J. Am. Ceram. Soc. 93 (2010) 4087-4095.
-

 This Article
This Article
-
2020; 21(3): 338-342
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.338
- Received on Feb 16, 2020
- Revised on Mar 18, 2020
- Accepted on Mar 20, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Guoguang Yao
-
School of Science, Xi’an University of Posts and Telecommunications, Xi’an 710121, China
Tel : +86 29 88166089
Fax: +86 29 88166333 - E-mail: yaoguoguang@xupt.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.