- Enhanced TE performance of FeVSb1-xSnx half-heusler matrices using zirconia vial
Rahidul Hasana,b, Kyu Hyoung Leeb and Soon-Chul Ura,*
aDepartment of Materials Science and Engineering / ReSEM, Korea National University of Transportation (KNUT), Chungju, Chungbuk 27469, Republic of Korea
bElectronic Device and Materials Laboratory (EDML), Yonsei University, Seoul 03727, Republic of Korea
Thermoelectric and transport
properties of FeVSb1-xSnx (0.015
Keywords: Thermoelectric, X-ray Diffraction, Lattice Thermal Conductivity, Scanning Electron Microscope, Mechanical Alloying
Mechanical alloying (MA) process is a well-known high
energy milling technique that can produce ultrafine
microstructures which can be controlled during the progress of the milling [1].
It has superior advantages over other traditional processes; for instance, it
may help to produce alloys that are difficult to produce applying
conventional metallurgical techniques, namely casting and
forging [2, 3]. Regarding this, MA can be utilized for elements with a low
melting point which may suffer from sublimation during processing.
Despite these benefits, there are few drawbacks of the MA process
which may generate problems during the synthesizing process. One important
effect that we suffered previously was the contamination of the
foreign elements from stainless-steel vial during the milling
[4]. It made the desired half-Heusler (HH) system unstable,
causing a negative doping effect. To overcome such
problems, zirconia vial was used in order to prevent the incorporation of
foreign elements from the vial.
Thermoelectric (TE) generators open a feasible way to
reduce the dependency of fossil fuels and greenhouse gas
emissions by converting waste heat into electricity [5, 6]. However, the
large-scale applications were not possible yet because of
moderate conversion efficiencies and high cost of the materials [7,
8]. Generally, the TE material performance is derived from, ZT = (α2σT)/(κlat.+κel.)
= (PF/κ)T . It is
obvious that an efficient TE material should have high α (Seebeck
coefficient), σ (electrical conductivity) but low κ
(thermal conductivity), κlat. (lattice
thermal conductivity) and κel. (thermal conductivity
due to electronic contribution) at the applicable temperature, T.
However, it is highly challenging to get high ZT because of
the inter-relation of these ZT parameters [9].
Half-Heusler (HH) alloys are the new addition in renewable
energy materials and could become an appropriate candidate for TE devices. HH
materials have advantages over other TE materials because of its
high-temperature strength, cheap ingredients and ease of synthesis process. HH
alloys showed improved TE efficiency from intermediate to high-temperature
waste heat conversion [10]. Generally, HH has considerable PF at 300 K. It is
the major merit point of getting high efficiency. Till today, the best TE
efficiency was observed from the HH alloys of ZrNiSn, ZrCoSb, and NbFeSb in the
range of ZT = 1~1.5 [11-15]. Ferluccio et al. showed that NbCoSb HH
also produced ZT ≈ 1 when it occupied vacancies at the Nb site [16].
Zhu et al. revealed that TE efficiency in HH alloys was limited due to large
lattice thermal conductivity (κlat.) around
10~13 Wm-1K-1
at 300 K [17]. Recently, FeVSb HH alloy has gained research
interest owing to its high PF value though it had a relatively high κ
value around 8~10 Wm-1K-1 [18].
Hence, the reduction of κ is one of the main objectives to get high ZT in FeVSb
alloys. For achieving optimum efficiency, doping in the FeVSb
HH system may reduce the thermal conductivity barrier [19]. Mass
scattering process may be one of the efficient ways
to reduce thermal conductivities in the HH system which may
reduce the κlat. around 4~5 Wm-1K-1
[20]. Recently, Zou et al. stated that κ could be reduced by
boundary scattering engineering [21]. Stadnyk et al. studied the Fe1-xCuxVSb
matrix and found metallic conductivity at elevated temperature owing to a
sequential-shift in Fermi level to the conduction band [22]. Some of the
previously reported highly efficient thermoelectric materials are p-type
ZrCoBi0.65Sb0.15Sn0.20 half-Heusler
(ZT ≈ 1.42 at 973 K) [23], p-type endoaxially
nanostructured PbTe (ZT ≈ 2.2 at 915K) [24], Bismuth-doped GeTe
(ZT ≈ 1.8 at 722 K) [25], Skutterudite DDyFe3CoSb12 (ZT > 1.3
at 773 K) [26] and Bismuth Chalcogenides, Bi2Te3/Sb2Te3
super-lattice (ZT≈2.4 at 300 K) [27].
For the investigation of the TE properties of FeVSb1-xSnx (0.015
FeVSb1-xSnx HH systems were
synthesized by MA process using the stoichiometric powder mixtures of Fe (63
µm), V (75 µm), Sb (45 µm) and Sn (63 µm) and all the powders were 99.9% pure.
MA process was carried out using a zirconia vial in a vibratory mechanical
mill for 6 h. The Speed of the mill was kept constant at 1,080 rpm throughout
the process. 5 mm diameter zirconia ceramic balls were used to
avoid contamination. A 325-mesh dry sieve was used for the sieving of
MAed powders. Consolidation of the MAed powders was carried out using VHP at 70
MPa pressure and at 1173K temperature for 2 h. Every process was carried out
under Ar-atmosphere to avoid contamination.
A particle size analyzer (PSA) was applied to get the
particle size and the data were confirmed by SEM. Phase transformation of MAed
powders and VHPed samples were studied by XRD. A scanning electron microscope
(SEM) was employed to analyze the microstructures of
the specimens. α and σ were measured by the 4-probe
method using ZEM-3. 3×3×10 mm3 rectangular samples were prepared for
conducting properties measurement and 10Φ×1 mm spherical disk was for
thermal diffusivity measurements. Heat transfer was captured by a laser flash
instrument using a TC-9000H. The density of the specimens was calculated on the
basis of the Archimedes principle. Hall measurements were taken
by the instrument Modified Keithley 7065 (USA) using
the Van der Pauw method. Rietveld refinement plot was produced using the py-GSAS-II program.
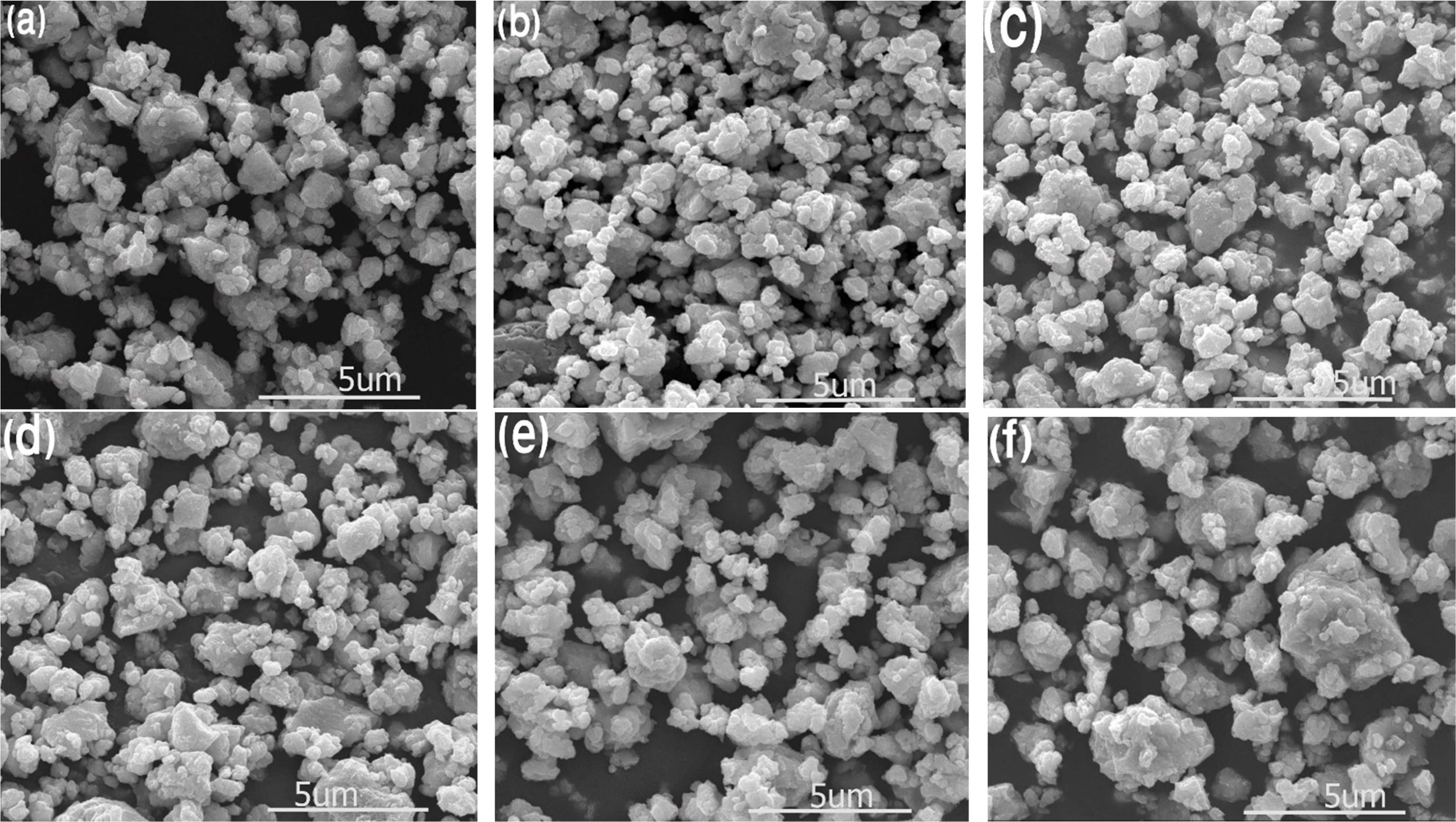
Generally, the particle size of a sample decreases when
doped with elements of a smaller ionic radius, which replaces the larger ions
from the specimen [28]. As-MAed powders are shown to be
near-spherical shape as in typical MA process and approximated particle
sizes are found to be less than 10 µm (Fig. 1). There is no considerable change
of particle morphology that might be due to the use of fractional
doping concentration.
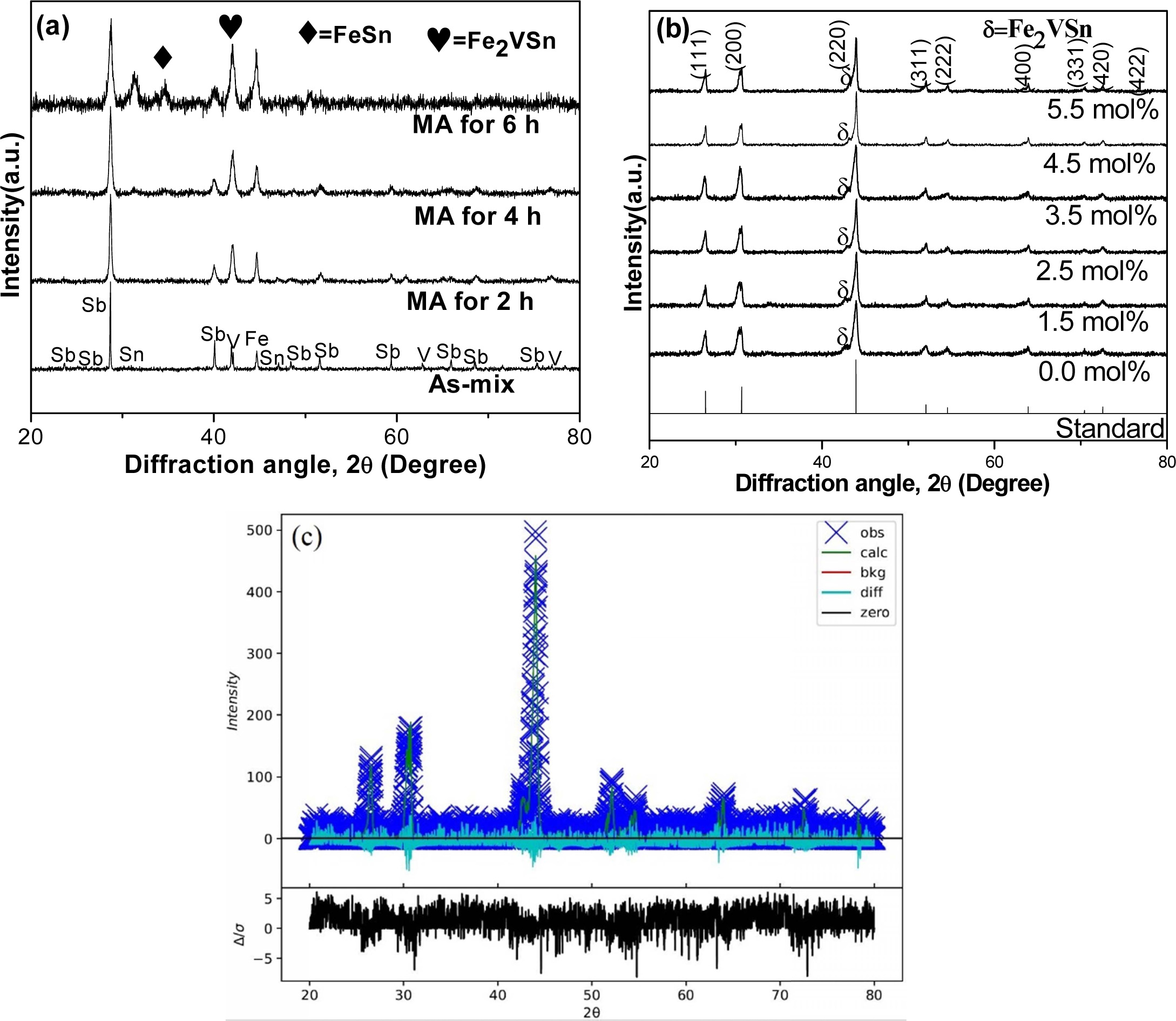
XRD patterns during milling are shown in Fig. 2(a) with
respect to time, and Fig. 2(b) shows the XRD curves of VHPed samples
synthesized by the MA process using zirconia vial. HH phases were coming out
after 4 h of milling and the remnant part of the HH phase developed during VHP
[29]. Two-second phases of FeSn and Fe2VSn were found in as-milled
powders. Among these second phases, FeSn disappeared; however,
Fe2VSn still remained after VHP. Near single HH phases
are dominating in VHPed samples though a fraction of the
second phase (Fe2VSn) remains, which is in agreement with our
previous study [4], (Fig. 2(b)). The Rietveld refinement of the bulk samples
(Fig. 2(c)) also confirms the presence of single-phase with F43m
symmetry.
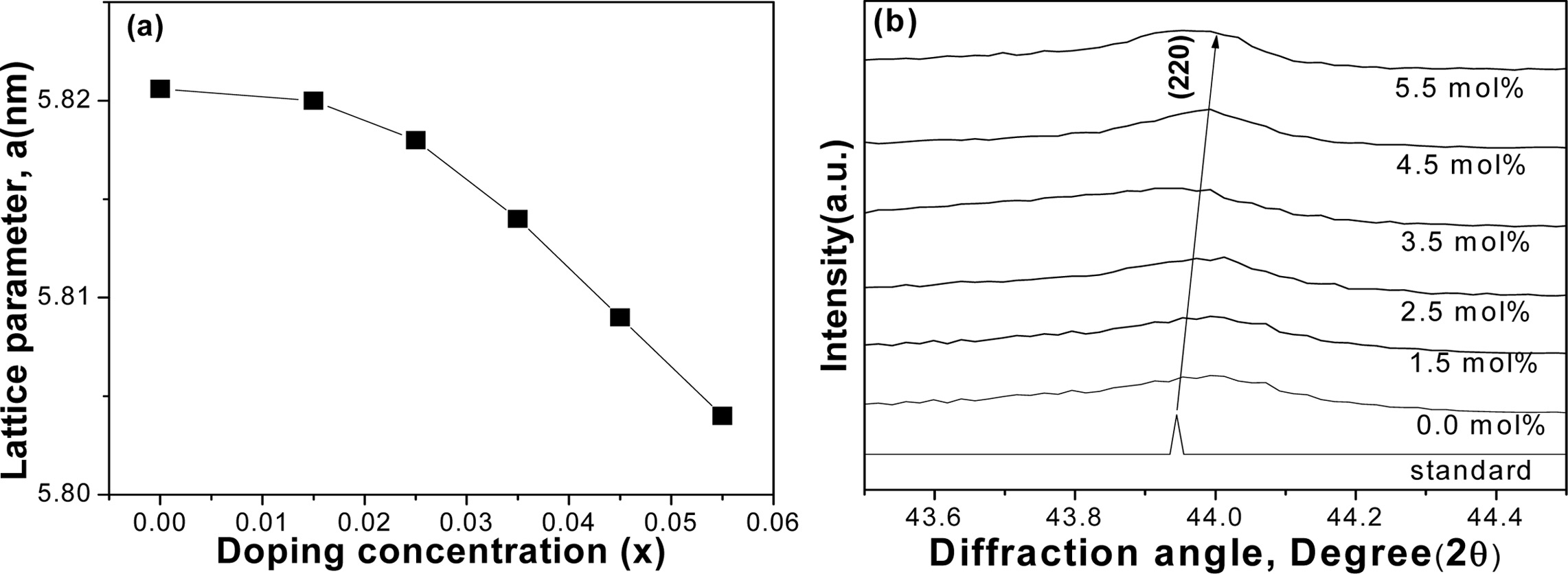
Lattice parameters of the various compositions are
depicted in Fig. 3(a). It is quite evident that the lattice parameters were
slightly decreased with the increase of Sn concentrations. Generally,
decreasing of lattice parameters could shift the diffraction peak (220) to the greater
angle [21]. The substitution of smaller Sn+4 (0.69 Å)
from the larger Sb+3 (0.76 Å) might be responsible for this effect,
as shown in Fig. 3(b). The second phase might have
played a role to decrease the lattice constant with the
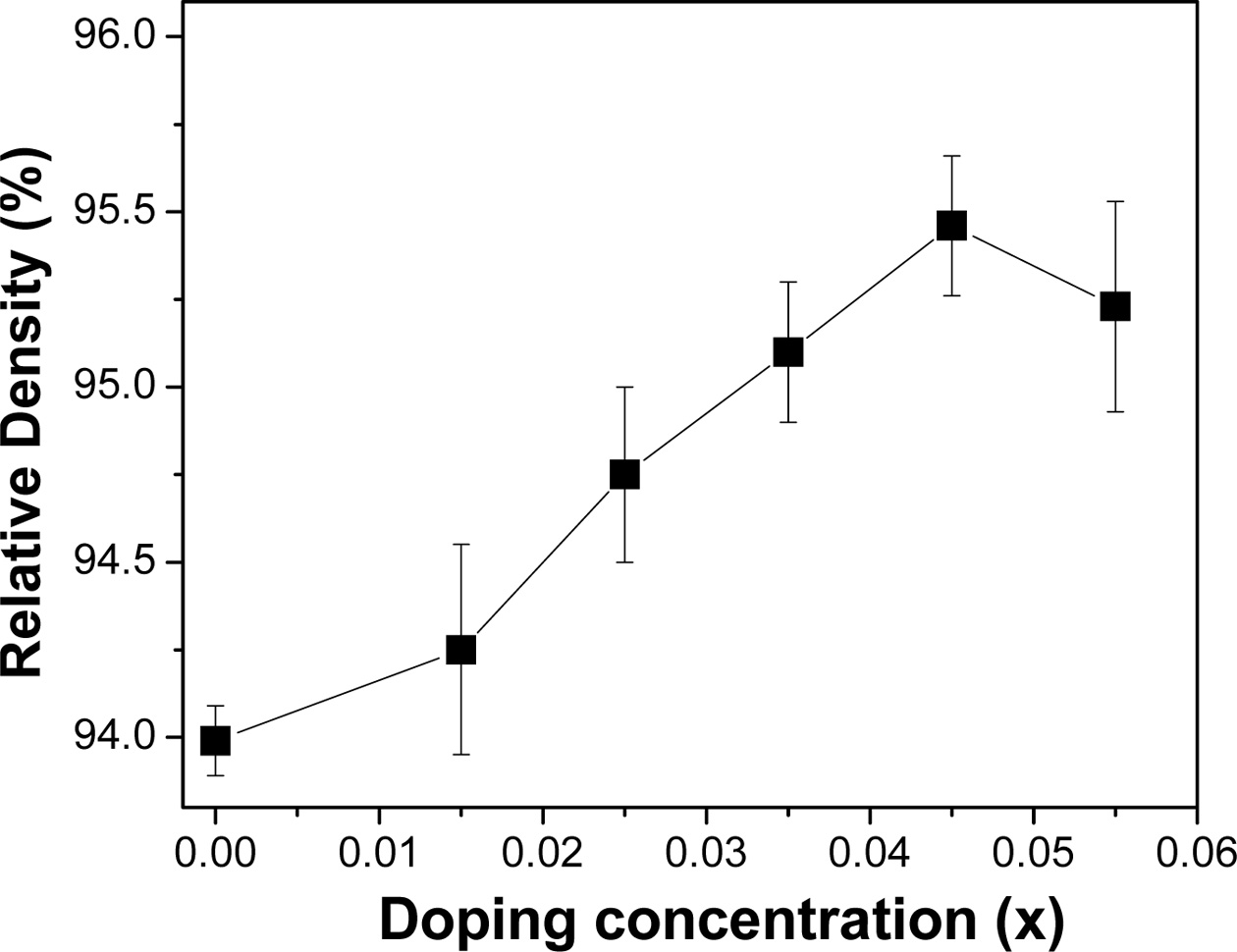
increase of Sn concentration [30]. The relative density is found to be quite
lower than our expectation (~95%, Fig. 5.). It might be due to the formation of
a small fraction of voids during hot-consolidation.
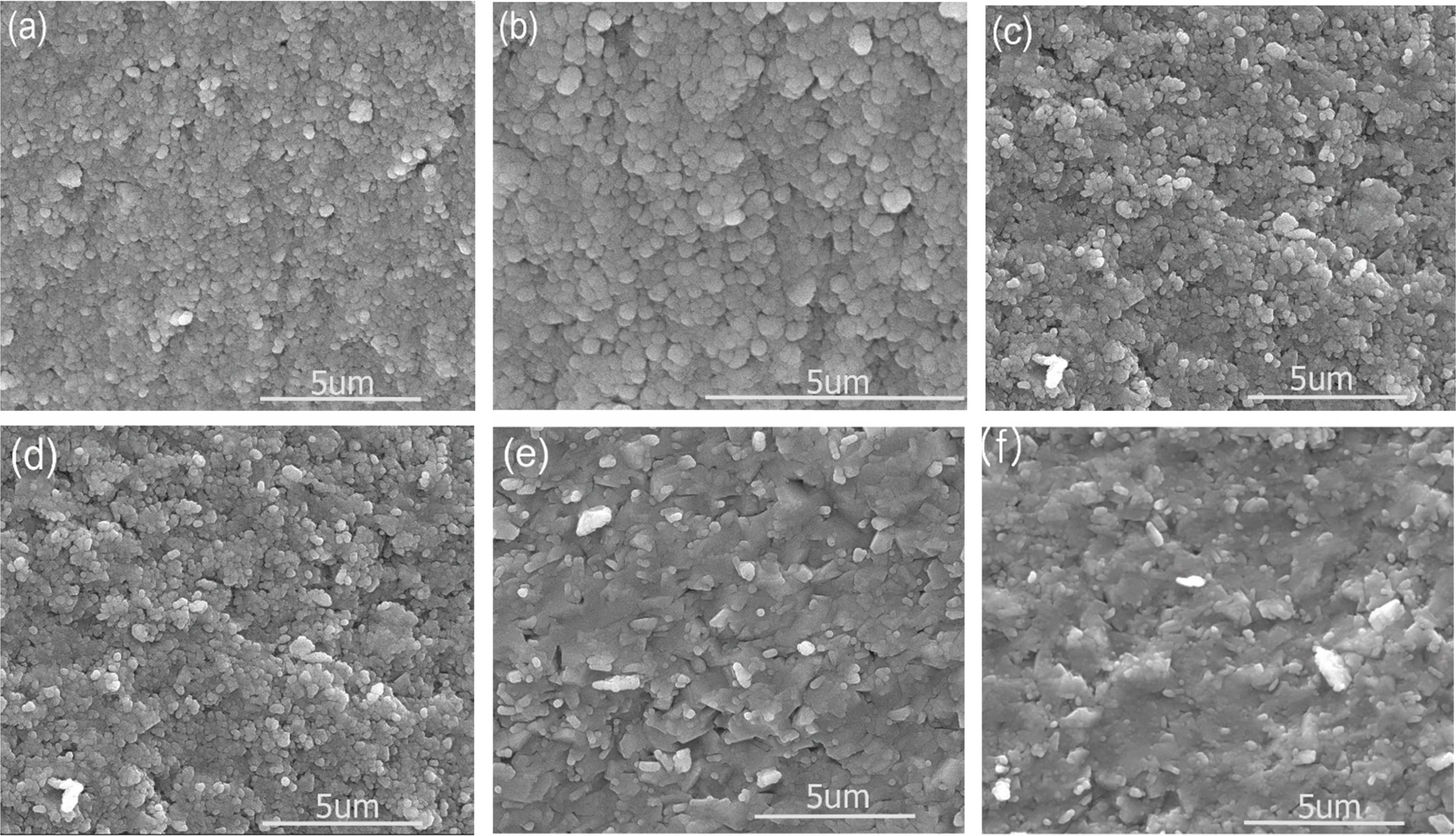
SEM images for VHPed samples are represented by Fig. 4.
Generally, the MA process gave rise to ultrafine microstructure [1]. In this
study, the average grain size could not be detected accurately from
the microstructures. However, the microstructures showed
that the approximate grain/particle size could be less than 10 µm, which
is a usual feature of MA and VHP [31]. From the SEM images, it
can be presumed to be prior particle boundaries or grain
boundaries present in the samples but we are not sure which one it is in this
set of experiments. Since the doping concentration used was very low, it didn’t
show any significant change in grain morphology as well.
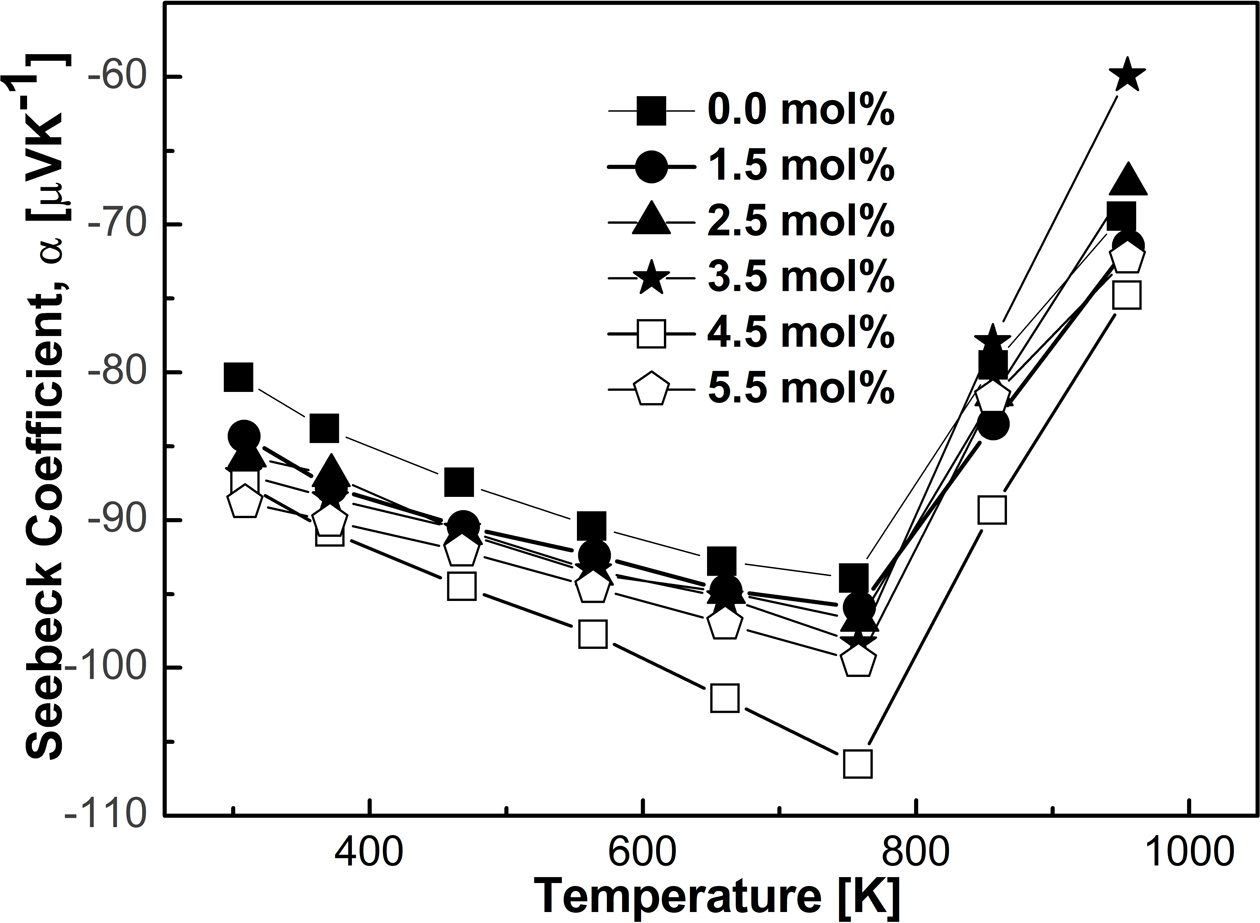
Fig. 6 illustrates the Seebeck coefficient of the VHPed
samples produced by the MA process using zirconia vial. The values of α
were detected negative in sign, which in turn reveal that the present charge carriers
were electrons. Notice that, the absolute value of α increases with
increasing doping concentration at 300 K in the MA process using zirconia vial.
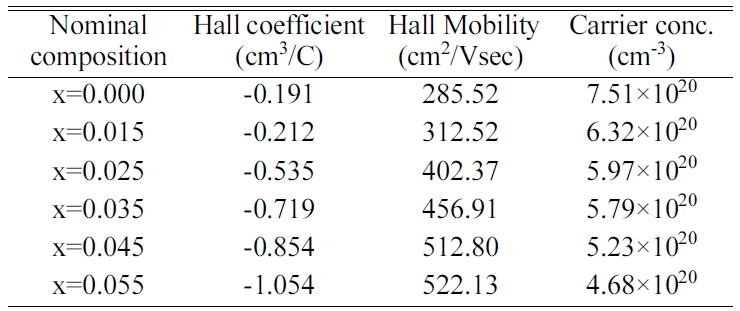
The Hall measurement data from Table 1 also supports this increase in the
absolute value of α. Generally, α increases with the decrease of
carrier concentration. On the contrary, the absolute value of α
decreased with increasing Sn contents during milling, possibly due to the
undesired incorporation of vial composition in the MA process using
stainless-steel vial [4], which was confirmed by EDS analysis. Shashanka and
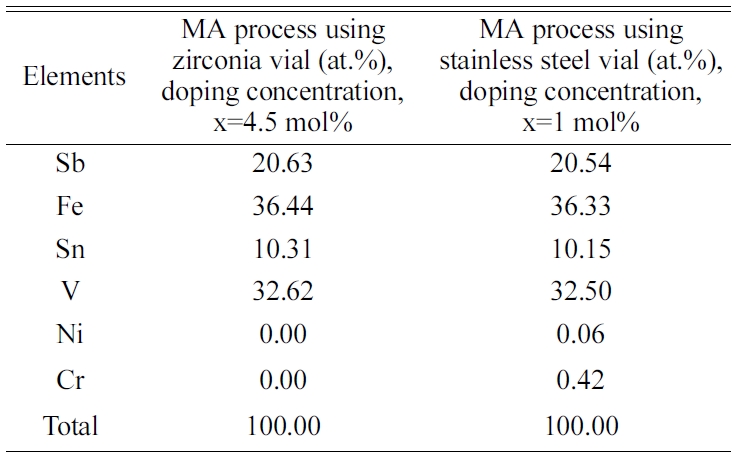
Chaira et al. also reported such incorporation in their work [32]. There
was no evidence of incorporation of the foreign elements from vial found in
controlled MA process using zirconia vial in this study, which is shown in Table
2. Moreover, the absolute value of α was increased with
increasing T and with doping concentration in the controlled MA process
using zirconia vial possibly owing to the intrinsic excitation of the bands
[33, 34]. The highest absolute value of α was found to be 106 µVK-1
at 758 K, which was observed for 4.5 mol% in the MA process using zirconia vial
indicated well-controlled composition of HH phase.
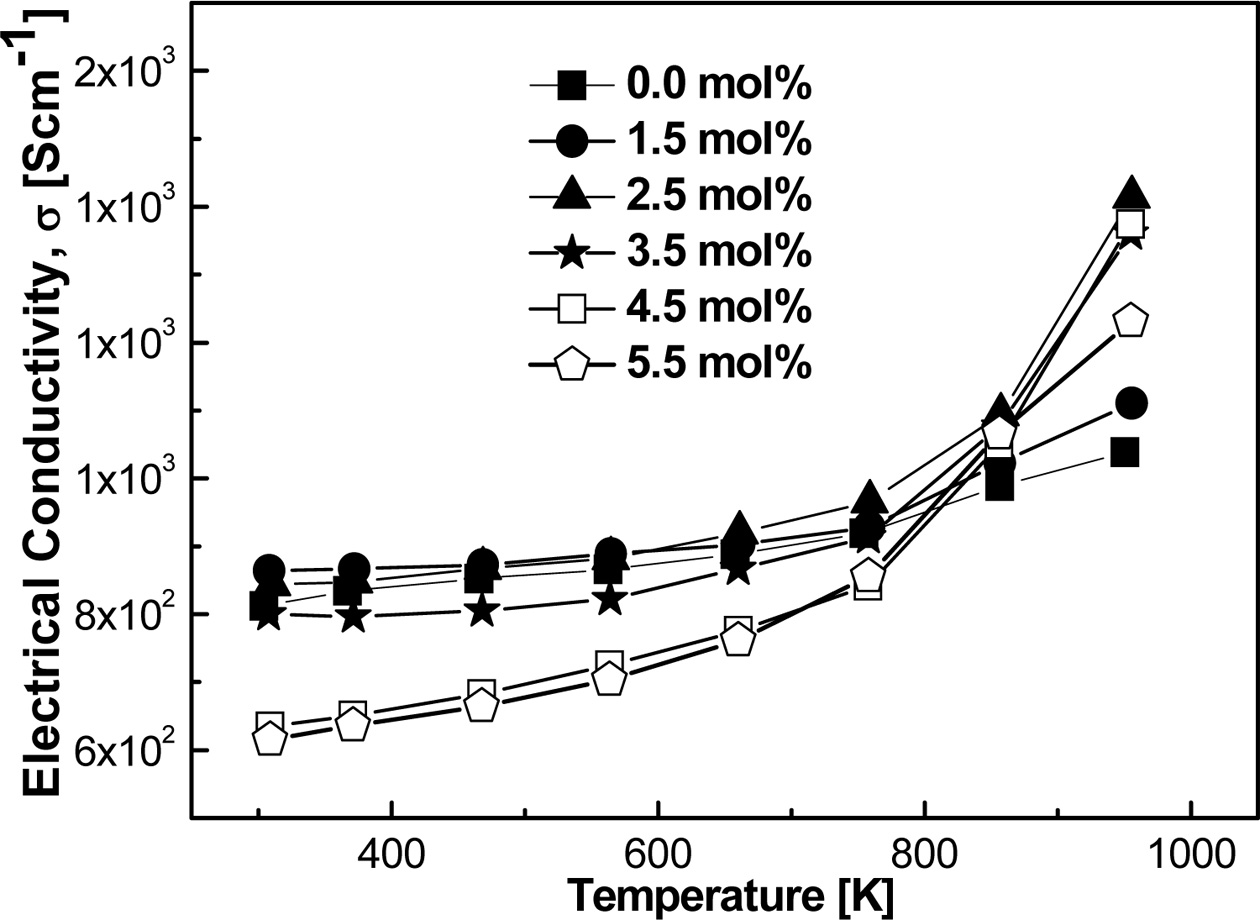
Fig. 7 showed the measured σ for controlled MA
process. The values of σ showed a similar trend to our previous work [4]
after adding dopants. σ was found to be decreased in the MA process
using both zirconia and stainless-steel vial with increasing Sn contents.
In both
cases, σ increased with increasing T, which indicated the inherent semi-metallic behavior.
This increasing trend of σ might have forced the Fermi band to the higher conduction band by the thermal
excitement of charge carriers within
the bandgap [35].
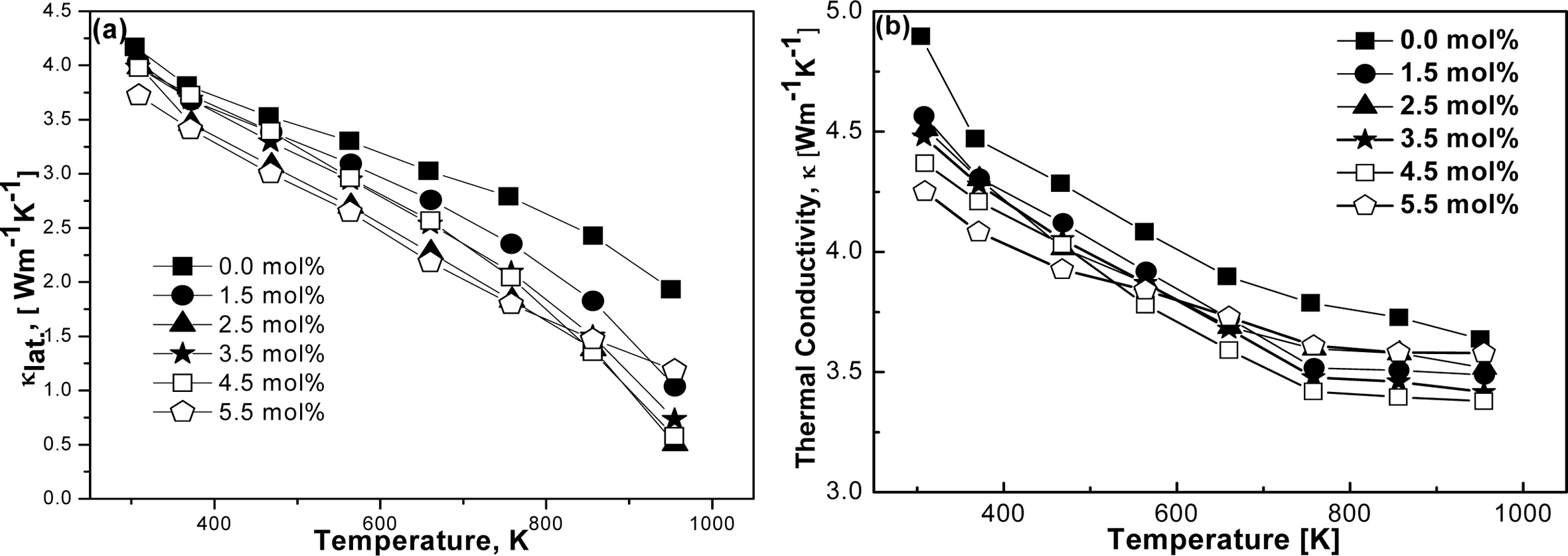
Fig. 8(a) and 8(b) represent κlat. and
κ with respect to time and doping elements.
Thermal energy is transferred by the thermalized electrons in the
conduction band and lattice vibration (phonon). The total thermal conductivity
of a material system can be expressed by the following equation, κ = κlat.
+ κel. Both κ and κlat.
of FeVSb1-xSnx were found to be decreased significantly with
increasing Sn contents. Possibly, enhanced phonon scattering
could play a major role in this decrease of κ and κlat.
[36]. Additionally, highly dense grain boundaries
might form because of the expected small grain size (~10 µm) [37].
Consequently, the scattering of phonon could take place at grain boundaries
which might produce a favorable path to the reduction of κlat..
A similar tendency was experienced for κ of all FeVSb1-xSnx
HH samples. There is a slight difference found between κ and κlat.
due to the very low value of κel. of FeVSb1-xSnx
HH alloys. Though κlat. is found considerably low; however,
it cannot be ignored for good TE devices. A portion of the second phase could
also be responsible for this decreasing value of κ [38]. The origin of
the cause in the differences of thermal conductivity
and lattice thermal conductivity might be the reduction
of the acoustic phonon bandwidth, consequently lowering the acoustic phonon
group velocities [39], which might decrease the lattice thermal conductivity.
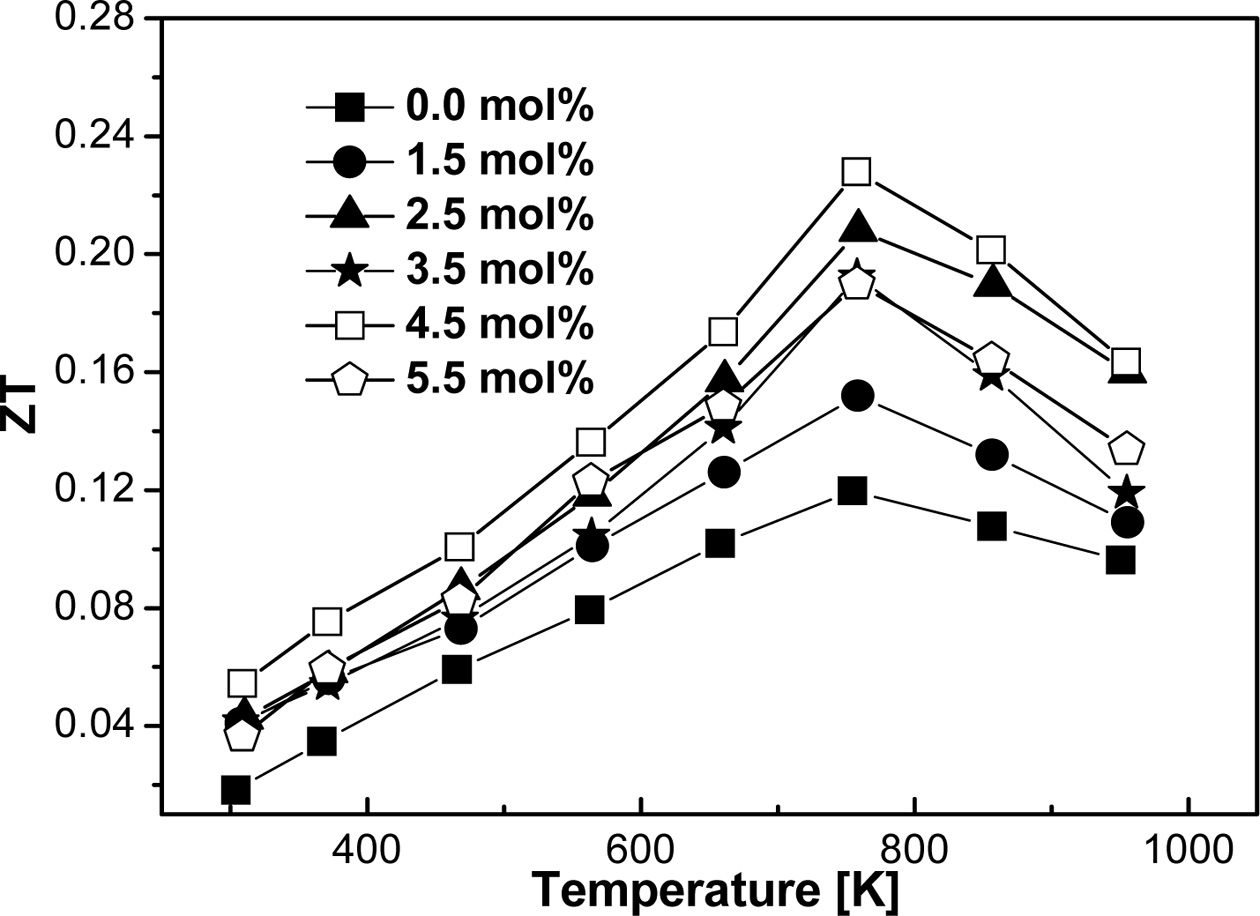
Fig. 9. indicates the ZT value calculated from α, σ,
and κ for the MA process using zirconia vial. Comparing the
MA process between zirconia vial and stainless-steel vial, the former produced
a much higher ZT compared to ref. [4], which is clearly seen from the figure.
Generally, a sample with high relative density possesses the maximum ZT and
that is evident for FeVSb0.955Sn0.045 at 757 K.
The corresponding ZTmax≈0.23 was
calculated because of relatively high α and low κ, which suggest
that ZT can be further improved by careful tuning of appropriate dopant. If κ
might have reduced further by applying multiple substitutions and/or small
grain formation, ZT could be enhanced to unity which is in good accordance with
Codrin et al. [25].
|
Fig. 1 Microstructures of powder specimens mill for 6 h; (a) 0 mol%, (b) 1.5 mol%, (c) 2.5 mol%, (d) 3.5 mol%, (e) 4.5 mol%, and (f) 5.5 mol%. |
|
Fig. 2 (a) XRD patterns for FeVSb0.945Sn0.055 during milling which represents other samples, (b) FeVSb1-xSnx (x=0.0~0.055) HH phases applying MA process using zirconia vial, and (c) representative Rietveld refinement of bulk sample x=4.5 mol%. |
|
Fig. 3 Crystal structure variations of VHPed samples; (a) lattice parameter, and (b) peak shifting to the greater diffraction angle. |
|
Fig. 4 Scanning electron microscope images of the various Sn-doped VHPed samples: (a) 0 mol%, (b) 1.5 mol%, (c) 2.5 mol%, (d) 3.5 mol%, (e) 4.5 mol%, and (f) 5.5 mol%. |
|
Fig. 5 The relative density of the bulk FeVSb1-xSnx HH systems against the doping concentration |
|
Fig. 6 The Seebeck coefficient of FeVSb1-xSnx HH systems as a function of T, processed by MA process using zirconia vial |
|
Fig. 7 Temperature dependence of σ values as a function of T for the MA process using zirconia vial |
|
Fig. 8 Calculated (a) κlat., (b) κ of the VHPed samples produced by MA process using zirconia vial. |
|
Fig. 9 Calculated ZT of VHPed FeVSb1-xSnx specimens for the MA process using zirconia vial. |
|
Table 1 Measured Hall data of the bulk FeVSb1-xSnx HH systems at room temperature synthesized by using zirconia vial. |
|
Table 2 EDS analysis of the VHPed samples synthesized by the MA process using zirconia and stainless-steel vial. |
FeVSb1-xSnx (x=0.015~0.055) HH
compositions were fabricated by the MA process using zirconia vial. A
subsequent VHP was required to consolidate the powders. After MA and VHP, all
the compositions produced near single HH phases. In this study, TE properties
of the MAed materials processed using zirconia vial and stainless-steel vial
were evaluated as a function of doping concentration and T and compared between
them. It was found that the incorporation of foreign elements from
stainless-steel vial reduced the absolute value of α and increased the κ
by negative doping effect. On the other hand, α was not being affected
by impurities in the MA process using zirconia vial, leading to a relatively
higher absolute value of α of 106 µVK-1 at 758 K for
the optimum composition. κ reduced comprehensively for the MA process
using zirconia vial, possibly due to enhanced phonon scattering
due to doped with Sn and second phase interaction. It was observed that
Sn-doping in FeVSb HH phases synthesized by the MA process using zirconia vial
reduced κ to its lowest value of 3.38 Wm-1K-1 from
intrinsic FeVSb (~8 Wm-1K-1).
A ZTmax of 0.23 was being calculated for FeVSb0.955Sn0.045
at 757 K using zirconia vial, which is a
much-improved value compared to the MA process using
stainless-steel vial [4]. The lower κlat. and relatively high
magnitude of α played the main part to reach this ZTmax.
Further improvements in TE efficiency it might be possible by the multi-doping
technique.
This research was supported by the Korea Basic Science
Institute grant funded by the Ministry of Education (grant no.
2019R1A6C1010047).
- 1. S.-C. Ur, H. Choo, D.B. Lee, and P. Nash, Metals and Materials. 6[5] (2000) 435-440.
-

- 2. M. Blair and T.L. Stevens, in “Steel Castings Handbook” (ASM International, Ohio, USA, 1995) p.71.
- 3. E.P. DeGarmo, J.T. Black and R.A. Kohser, in “Solution Manuals to Accompany-Materials and Process in Manu- facturing” (John Wiley and Sons Ltd, 2003) p.122.
- 4. R. Hasan and S.-C. Ur, J. of Trans. Electr. Electron. Mater. 19[2] (2018) 106-111.
-

- 5. D.M. Rowe, in “Thermoelectrics and Its Energy Harvest- ing” (CRC Press, 2012) p.80.
- 6. T.M. Tritt, Annu. Rev. Mater. Res. 41[1] (2011) 433-448.
-

- 7. M. Zebarjadi, K. Esfarjani, M.S. Dresselhaus, Z.F. Ren, and G. Chen, Energy Environ. Sci. 5[1] (2012) 5147-5162.
-

- 8. W.S. Liu, Q. Jie, H.S. Kim, Z.F. Ren, Acta Mater. 87 (2015) 357-376.
-

- 9. G.J. Snyder and E.S. Toberer, Nature Materials. 7[2] (2008) 105-114.
-

- 10. S.A. Barczak, J. Buckman, R.I. Smith, A.R. Baker, E. Don, I. Forbes, and J.W.G. Bos, Materials. 11[4] (2018) 536.
-

- 11. L. Chen, S. Gao, X. Zeng, A.M. Dehkordi, T.M. Tritt, and S.J. Poon, Appl. Phys. Lett. 107[4] (2015) 041902.
-

- 12. M. Gurth, G. Rogl, V.V. Romaka, A. Grytsiv, E. Bauer, and P. Rogl, Acta Mater. 104 (2016) 210-222.
-

- 13. X.A. Yan, G. Joshi, W.S. Liu, Y.C. Lan, H. Wang, S. Lee, J.W. Simonson, S.J. Poon, T.M. Tritt, G. Chen, and Z.F. Ren, Nano Lett. 11[2] (2011) 556-560.
-

- 14. E. Rausch, B. Balke, T. Deschauer, S. Ouardi, and C. Felser, APL Mater. 3[4] (2015) 105.
-

- 15. C.G. Fu, S.Q. Bai, Y.T. Liu, Y.S. Tang, L.D. Chen, X.B. Zhao, and T.J. Zhu, Nature Communications. 6[1] (2015) 6888.
-

- 16. D.A. Ferluccio, R.I. Smith, J. Buckman, and J.W.G. Bos, Phys. Chem. Chem. Phys. 20[6] (2018) 3979-3987.
-

- 17. T.J. Zhu, C.G. Fu, H.H. Xie, Y.T. Liu, and X.B. Zhao, Adv. Energy Mater. 5[19] (2015) 1500588.
-

- 18. A. Yamamoto and T. Takeuchi, J. of Elec. Materi. 46[5] (2017) 3200-3206.
-

- 19. H. Hohl, A.P. Ramirez, C. Goldmann, G. Ernst, B. Wolfing, and E. Bucher, J. Phys.: Condens. Matter. 11[7] (1999) 1697-1709.
-

- 20. L.D. Zhao, S.H. Lo, Y.S. Zhang, H. Sun, G.J. Tan, C. Uher, C. Wolverton, V.P. Dravid, and M.G. Kanatzidis, Nature. 508[7496] (2014) 373-377.
-

- 21. M. Zou, J.F. Li, and T. Kita, J. of Sol. Stat. Chem. 198 (2013) 125-130.
-

- 22. Y. Stadnyk, A. Horyn, V. Sechovsky, L. Romaka, Y. Mudryk, J. Tobola, T. Stopa, S. Kaprzyk, and A. Kolomiets, J. Alloys Compd. 402 (2005) 30-35.
-

- 23. H. Zhu, R. He, J. Mao, Q. Zhu, C. Li, J. Sun, W. Ren, Y. Wang, Z. Liu, Z. Tang, A. Sotnikov, Z. Wang, D. Broido, D.J. Singh, G. Chen, K. Nielsch, and Z. Ren, Nature Communications. 9[1] (2018) 2497.
-

- 24. K. Biswas, J. He, I. D. Blum, C.-I. Wu, T.P. Hogan, D.N. Seidman, V.P. Dravid, and M.G. Kanatzidid, Nature 489[7416] (2012) 414-418.
-

- 25. J. Li, Z. Chen, X. Zhang, Y. Sun, J. Yang, and Y. Pei, NPG Asia Mater. 9[3] (2017) e353.
-

- 26. G. Rogl, A. Grytsiv, R. Anbalagan, J. Bursik, M. Kerber, E. Schaflur, M. Zehetbauer, E. Bauer, and P. Rogl, Acta Mater. 159 (2018) 352-363.
-

- 27. R. Venkatasubramanian, E. Siiviola, T. Colpitts, and B. O’Quinn, Nature. 413[6856] (2001) 597-602.
-

- 28. I.-H. Kim, J.-C. Kweon, Y.-G. Lee, M.-S. Yoon, S.-L. Ryu, W.-G. Kim, and S.-C. Ur, Mater. Sci. Forum. 658 (2010) 33-36.
-

- 29. E. Yuasa, T. Morooka, M. Tsunoda, and J. Mishima, J. of the Japan Soc. of Powd. and Powd. Metall. 42[2] (1995) 171-174.
-

- 30. K.D. Codrin, T. Yamada, A. Yamamoto, R. Sobota, M. Matsunami, and T. Takeuchi, Jpn. J. Appl. Phys. 56[11] (2017) 111202.
-

- 31. P. Jozwik and Z. Bojar, Archives of Metallurgy and Materials. 52[2] (2007) 321-327.
- 32. R. Shashanka and D. Chaira, in “Ball Milled Nano-Structured Stainless-Steel Powders-Fabrication and Characterization” (Educreation Publishing, 2011) p.8.
- 33. H.J. Goldsmid, in "Electronic refrigeration" (Pion, 1986).
- 34. T. Xing, R. Liu, F..Hao, P. Qiu, D. Ren, X. Shi, and L. Chen, J. Mater. Chem. C, 5[47] (2017) 12619-12628.
-

- 35. D.P. Young, P. Khalifah, R.J. Cava, and A.P. Ramirez, J. of Appl. Phys. 87[1] (2000) 317-321.
-

- 36. J.J. Gong, A.J. Hong, J. Shuai, L. Li, Z.B. Yan, Z.F. Ren, and J.-M. Liu, Phys. Chem. Chem. Phys. 18[24] (2016) 16566-16574.
-

- 37. R. Hasan and S. C. Ur, Elect. Mater. Lett. 14[6] (2018) 725-732.
-

- 38. J. Cui, G. Cai, and W. Ren, RSC Adv. 8[38] (2018) 21637-21643.
-

- 39. H. Euchner, S. Pailhès, V. M. Giordano, and M. de Boissieu, Phys. Rev. B. 97[1] (2018) 014304.
-

 This Article
This Article
-
2020; 21(3): 319-325
Published on Jun 30, 2020
- 10.36410/jcpr.2020.21.3.319
- Received on Nov 7, 2019
- Revised on Mar 6, 2020
- Accepted on Mar 9, 2020
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Soon-Chul Ur
-
Department of Materials Science and Engineering / ReSEM, Korea National University of Transportation (KNUT), Chungju, Chungbuk 27469, Republic of Korea
Tel : +82-43-841-5385 - E-mail: scur@ut.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.