- Nano-crystallized glass-ceramic of BaO-TiO2-SiO2 system containing ZrO2
Byeong-Guk Kang and Seunggu Kang*
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon 16227, Korea
Fresnoite (Ba2TiSi2O8)
materials, which exhibit piezoelectricity, ferroelectricity, and nonlinear
optical properties, can be used as core materials for optical communication and
optical information processing devices. In this study, glass-ceramics with
nanometer size fresnoite crystal phase were prepared from BaO-TiO2-SiO2
(BTS) glass containing ZrO2 as a nucleating agent and their
microstructure, crystallization behavior, and photoluminescence properties were
analyzed. The heat treatment process for glass-ceramics was designed based
on data obtained by non-isothermal analysis using differential thermal analysis
(DTA). The crystal morphology and microstructure of the prepared specimens were
analyzed by X-ray diffraction analysis (XRD), optical microscopy (OM), and
field emission scanning electron microscopy (FE-SEM), respectively. The
luminescence properties of the specimens were analyzed by photoluminescence
(PL) measurements. When the BTS glass with 15 wt% ZrO2 (15Z)was heat
treated at 1,015 oC, a dendritic structure by gathering very
small spherical crystalline phases of 10-30 nm was formed. In addition, when
the 15Z was excited by light of 309 nm wavelength, strong light of 469 nm
wavelength was emitted, and this light appeared to be blue light close to white
in the Commission International de l'Eclairage (CIE) diagram.
Keywords: Glass-ceramic, Fresnoite, Nano-crystal, Photoluminescence
Glass-ceramic is a composite material in which crystals
are dispersed in a vitreous matrix. When the glass specimen is subjected to
sufficient thermal energy required for nucleation and crystal growth,
glass-ceramic is formed. Glass-ceramic has completely new electrical, optical,
mechanical, and chemical properties due to the crystalline phase produced in
the matrix as compared to the parent glass. In order to produce a glass-ceramic
having high functionality, the target crystal phase should be precipitated in a
vitreous state. For this purpose, the temperature and time of heat treatment in
the nucleation and crystal growth process must be strictly controlled.
Generally, it is known that nuclei generated in a glass-ceramic
do not grow epitaxially during crystal growth[1].
There are some glass compositions capable of forming nuclei
by themselves. However, in general, when a glass-ceramic is
manufactured, a nucleating agent is added to the glass-ceramic or droplets
induced by a liquid/liquid phase separation phenomenon in the glass serve as
nuclei. When the nuclei thus produced are subjected to heat
treatment at a high temperature, crystallization occurs to
precipitate target crystals [2]. Typical nucleating agents used in the
crystallization process include ZrO2, TiO2, P2O5,
CaF2, Cr2O3, Fe2O3, etc
[3]. TiO2 and ZrO2 nucleating agents are predominantly
used in silicate-based glasses. However, it is known that the addition of TiO2
decreases the crystallization temperature of the parent glass and the addition
of ZrO2 increases the crystallization temperature. Reben et al. also
published an interesting result that the addition of TiO2 to CRT
(Cathode Ray Tube) glass powder induces surface crystallization behavior, not
bulk crystallization [4]. Wondraczekw
et al. established optimal crystallization conditions by selecting TiO2
and ZrO2 to produce β-quartz crystal phases of 100 nm or less in LAS
(Li2O-Al2O3-SiO2) glass [5]. In
addition, by adding ZrO2 to the glass composition, Duke et al.
produced β-quartz crystals uniformly, thereby obtaining high transparency and
low expansion rate. Therefore, ZrO2 and TiO2 has been
widely conducted as nucleating agent [6].
On the other hand, it is known that fresnoite (Ba2TiSi2O8)
crystals exhibit piezoelectricity, ferroelectricity, and nonlinear optical
characteristics. When fresnoite is irradiated with ultraviolet light, blue
light is emitted due to Ti4+ ions present in the square-pyramidal
TiO5 structure. Recently, fresnoite-based glass-ceramics have been
attracting interest. Fresnoite-based glass-ceramics can be
applied to optical devices such as optical switches and optical
waveguides [7-11]. Lee et al. studied the fluorescence properties of BaO-TiO2-SiO2
(BTS) as a function of the ratio of K2O/BaO. Lee contended that the
degree of crystallinity and the PL intensity of glass-ceramics without K2O
are high, but that the degree of crystallinity and the photoluminescence (PL)
intensity decrease with an increasing K2O/BaO ratio [12].
Although various studies have been carried out on fresnoite-based
glass-ceramics exhibiting PL characteristics, it
is difficult to find any studies to induce bulk crystallization by adding the
ZrO2 nucleating agent. Therefore, in this study, ZrO2 was
added to BTS glass to induce bulk crystallization behavior, and also controlled to produce only nanaometer-sized fresnoite
crystal phase. Finally, the microstructure and the crystal phase of the
prepared glass-ceramic were analyzed and the results
were discussed in relation to the luminescence properties.
The starting materials were BaCO3 (Kojundo
Chemical Co., 99%), TiO2 (Kojundo Chemical Co., 99.9%),
SiO2 (Kojundo Chemical Co., 99.9%), and ZrO2 (Junsei
Chemical Co., 99%). The composition of the mother glass was fixed to BaO : TiO2
: SiO2 = 26: 13: 61 (mol%), which is
higher in the amount of SiO2 than the composition of the fresnoite
(Ba2TiSi2O8) phase, to
generate fresnoite crystals on the glassy matrix
in which SiO2 is the main component. To prepare
the glass-ceramic, ZrO2 was added to the mother glass in the range
of 0 to 15 wt%. The batch powder was pulverized and mixed with zirconia balls
as milling media for 24 h and then melted in an alumina crucible at 1,450 oC
for 1 h in an electric furnace. The melt obtained was quenched by pouring into
a graphite mold. The obtained glass was pulverized to a size of 45 μm or less
and then subjected to differential thermal analysis (DTA, STA S1500, Scinco
Co., Korea) at a heating rate of 5 oC/min to analyze the
crystallization mechanism. Table 1 shows the heat treatment process conditions
for glass-ceramics manufacturing based on the data obtained from the DTA
results. The crystalline phase of the glass-ceramic was confirmed by X-ray
diffraction analysis (XRD, Pan'alytical, X'pertpro, Netherlands), and the
crystallization behavior was con- firmed by optical microscopy
(OM, ME-33, Daemyoung Optical Co., Korea). The nanoscale
microstructure was confirmed with a field emission scanning electron microscope
(FE-SEM, S-4800, HITACHI Co., Korea). Before the observation, the fractured
surface of the glass-ceramic was polished by SIC sandpaper and
etched with 1 wt% hydrofluoric acid (HF) for 30 seconds. Luminescence
properties of glass-ceramics were observed by
photoluminescence and photoluminescence excitation
spectroscopies (PL and PLE, Darsa-5000, PSI Co., Korea) at room temperature. A
500 W xenon lamp was used as the excitation source and the emitted light was analyzed
using the Commission International de l'Eclairage (CIE)
diagram.
|
Table 1 One-step heat-treatment condition for fresnoite-based glass-ceramics. |
*Composition of mother glass is BaO: TiO2 : SiO2 = 26 : 13 : 61 (mol%) |
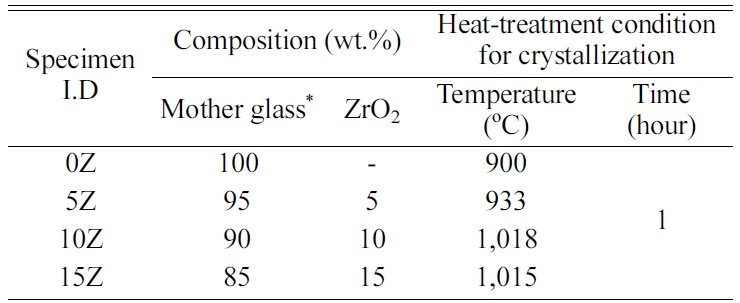
DTA analysis was performed on glass prepared by
substituting 0, 5, 10, and 15 wt% of nucleating agent ZrO2 in BTS
glass powder. The results are shown in Fig. 1. The maximum crystal growth
temperature (TP) is found to be 940 oC for the
mother glass (0Z), 973 oC for the 5Z specimen, 1,058 oC
for the 10Z specimen, and 1,045 oC for the 15Z specimen. That
is, when the ZrO2 substitution amount is in the range of 0-15 wt%, the TP value increases with the ZrO2
substitution amount, showing the highest value at 10% ZrO2, and decreases
when the ZrO2 substitution amount is over 10%.
Reben reported that the addition of ZrO2 as a nucleating agent to
fresnoite glass-ceramic tends to increase the crystal growth temperature [4]. However, when ZrO2
was replaced at more than 15wt% in this study, TP decreased. An
accurate interpretation of this tendency is expected to require additional
research.
We often have found that when glass-ceramics are
manufactured, the crystals grow beyond micrometer size due to too long heat
treatment or excessively high temperature than maximum crystal growth
temperature (Tp) obtained from DTA.
Therefore, glass was heated at a temperature of 40 oC
lower than Tp to produce a nano-sized fresnoite crystal phase. As shown
in Fig. 1, heat-treatment temperatures of the 0Z, 5Z, 10Z and 15Z specimens
were 900 oC, 933 oC, 1,018 oC
and 1,015 oC, respectively.
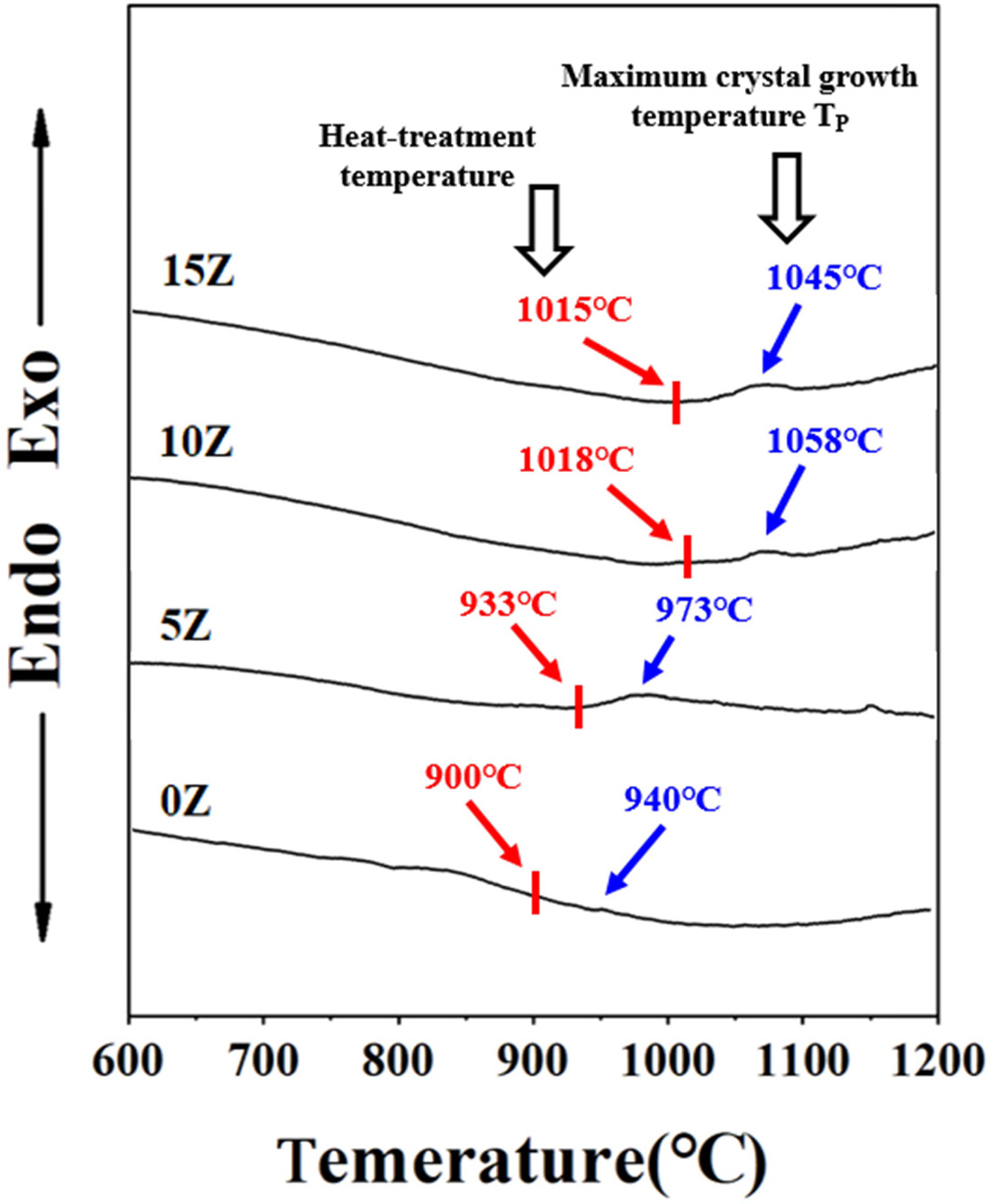
A graph of the XRD analysis results of the glass-ceramic
prepared according to the ZrO2 substitution amount is shown in Fig.
2. All glass-ceramics showed no or weak crystal
peaks, whereas the strong peaks for fresnoite crystals were formed in the specimen containing 10% ZrO2. The SiO2
might involve in the formation of both the
SiO2-based crystal phase and the amorphous matrix due to the
excessive amount of SiO2 added
compared to the stoichiometric fresnoite (Ba2TiSi2O8)
composition. Liu argued that when excess ZrO2
was added to the glass-ceramic, it would no longer function as a nucleating
agent but as a network former to strengthen the glass network,
and thus inhibits crystal growth [13]. Thus, the decrease in crystal peak intensity
of the 15Z specimen in the XRD graph is
believed to be due to the excess ZrO2 amount, acting as a
glass former rather than as a nucleating agent, as claimed by Liu.
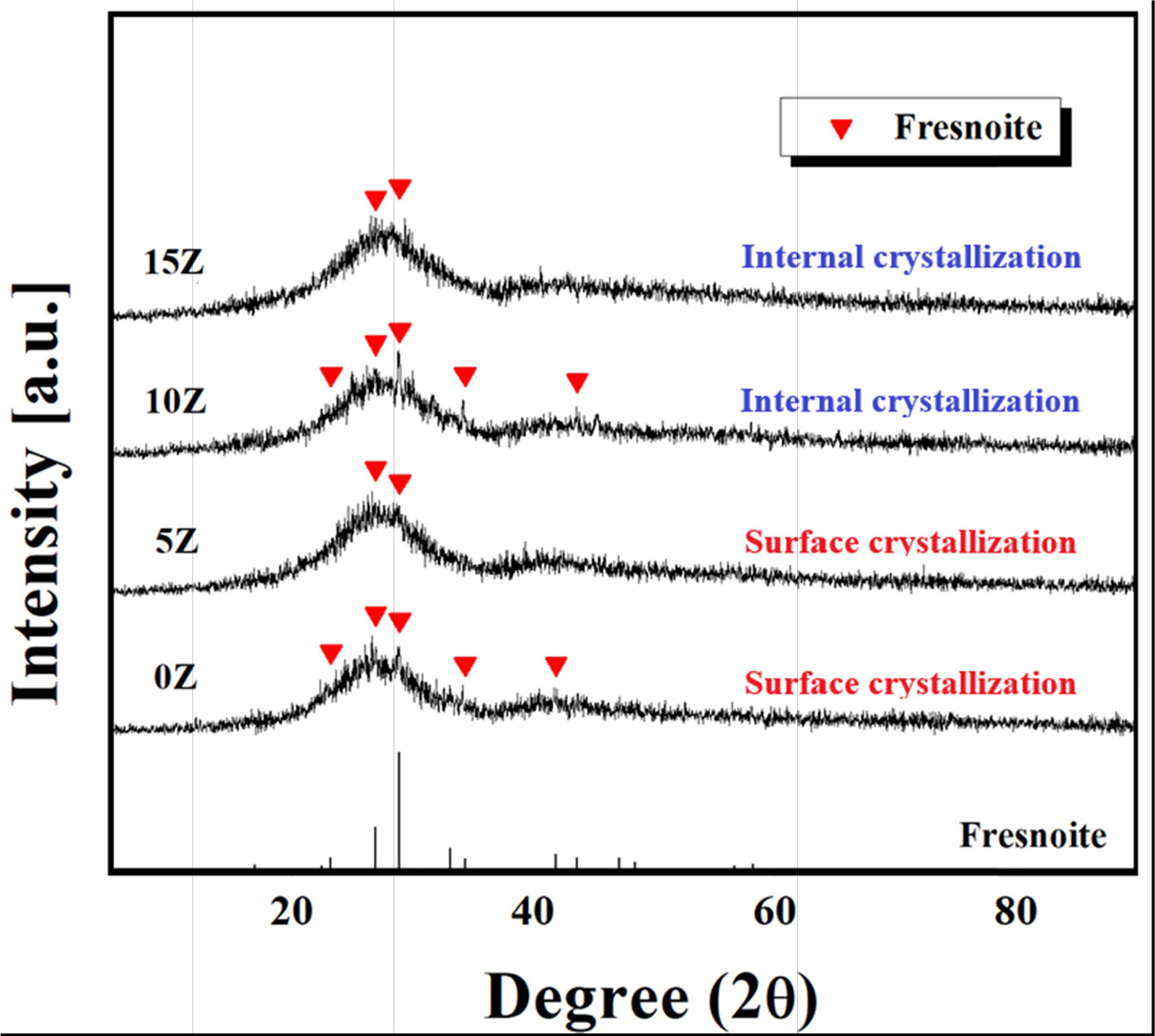
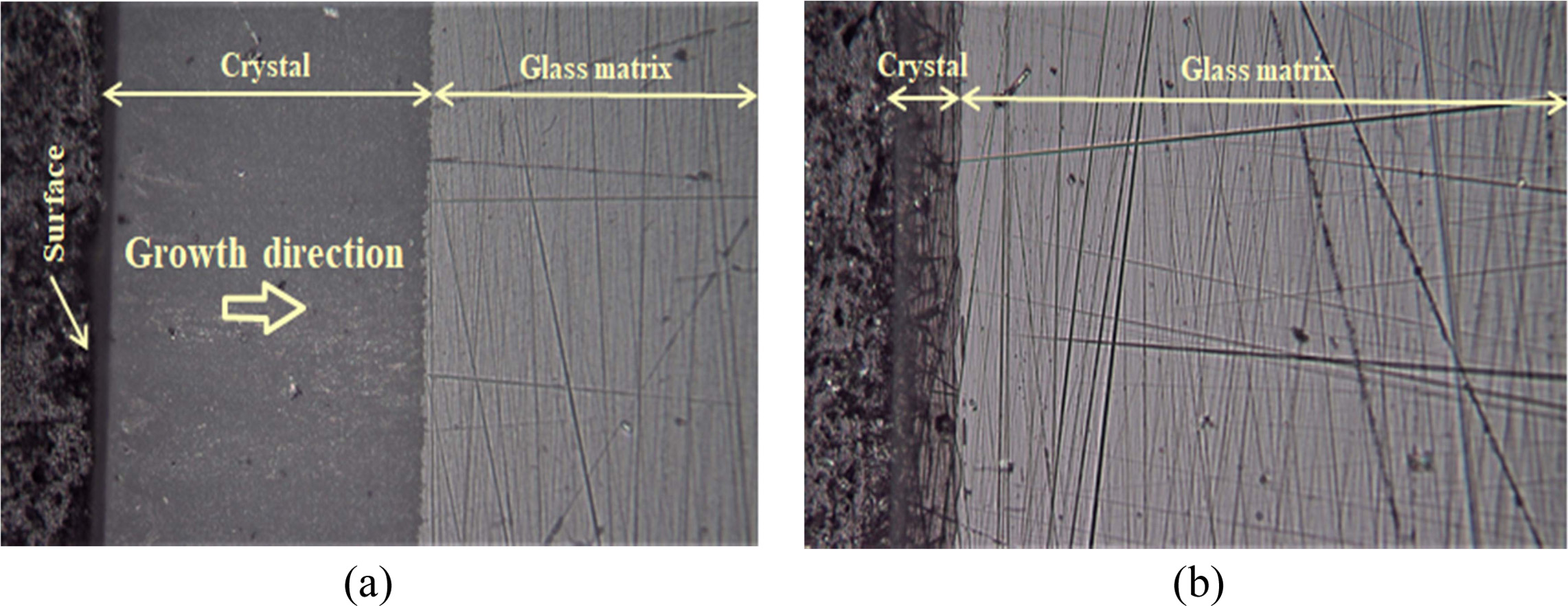
Also, as can be seen from the vertical section image shown in Fig. 3, surface crystallization phenomenon occurred in 0Z and 5Z specimens. Both specimens produced thick crystals on the surface, the thicknesses of surface crystal
of 217 μm and 46 μm, respectively. Due to the low hardness of the glass matrix, the crystal layer was
polished cleanly, whereas the glass matrix had many polishing marks. On the
other hand, in 10Z and 15Z specimens, no crystal layer
was observed on the surface, indicating that internal
crystallization occurred.
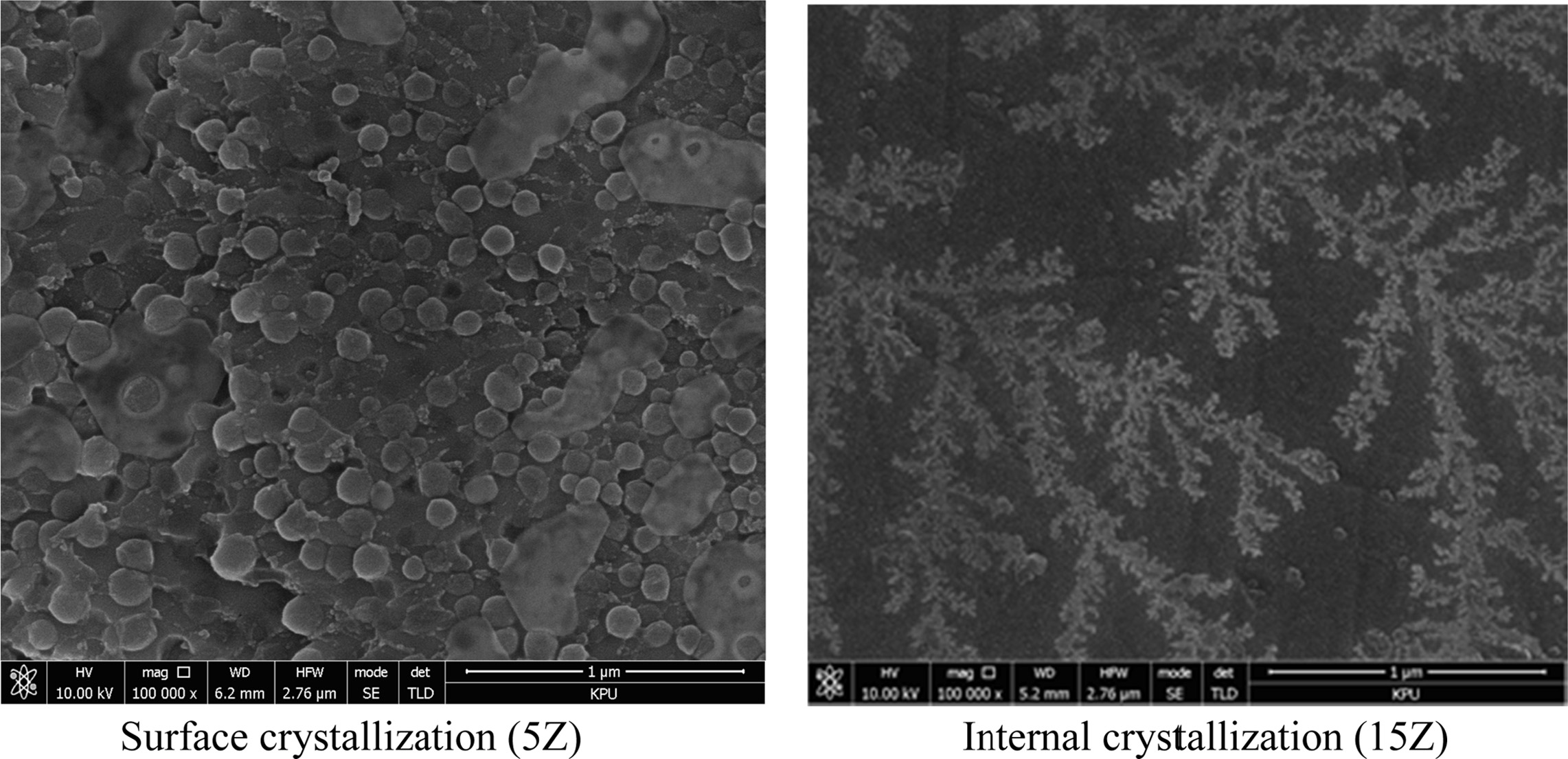
The microstructure of glass-ceramics prepared according
to the amount of ZrO2 substitution was observed by FE-SEM and the
results are shown in Fig. 4. The microstructure of the surface crystal part of
the 5Z specimen, magnified 50,000 times by SEM, was filled with spherical
crystals of 80-120 nm in size. On the other hand, the 15Z specimen
showed a dendrite-shaped crystal phase
composed of very small spherical particles of 10~30 nm
in size.
Wisniewski et al.
reported that several mechanisms are involved in the growth of
fresnoite crystals. Relatively slow growing crystals
form spherical or polygonal crystals, and
relatively fast growing crystals form dendrites [14-16]. In this study, the amount of ZrO2 added
determines the internal or surface crystallization, indicating that the crystallization rate varies
depending on the amount of ZrO2 added, and eventually the crystal
phase changes.
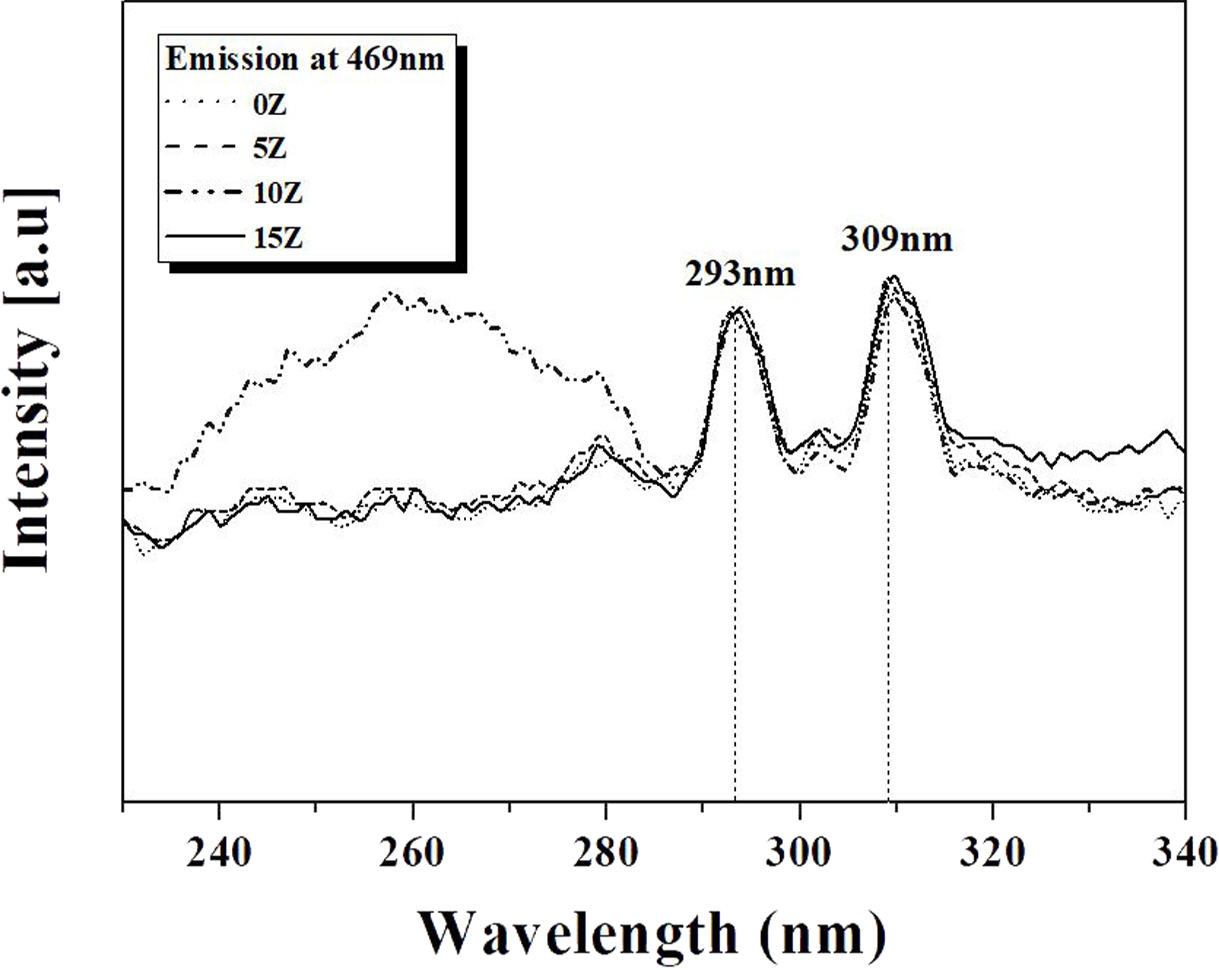
Photoluminescence excitation (PLE) and photo- luminescence
(PL)
analyses were performed to analyze the light emission characteristics of the
glass-ceramics prepared
according to the amount of ZrO2 added,
and the results are shown in Figs. 5 and 6, respectively. When
the light of 309 nm wavelength is irradiated to the glass ceramic specimen, the
light of 469 nm wavelength is emitted most as shown in Fig. 5. Therefore, when
analyzing the luminescence characteristics of the specimen prepared in this
study, 309 nm light was used as excitation light.
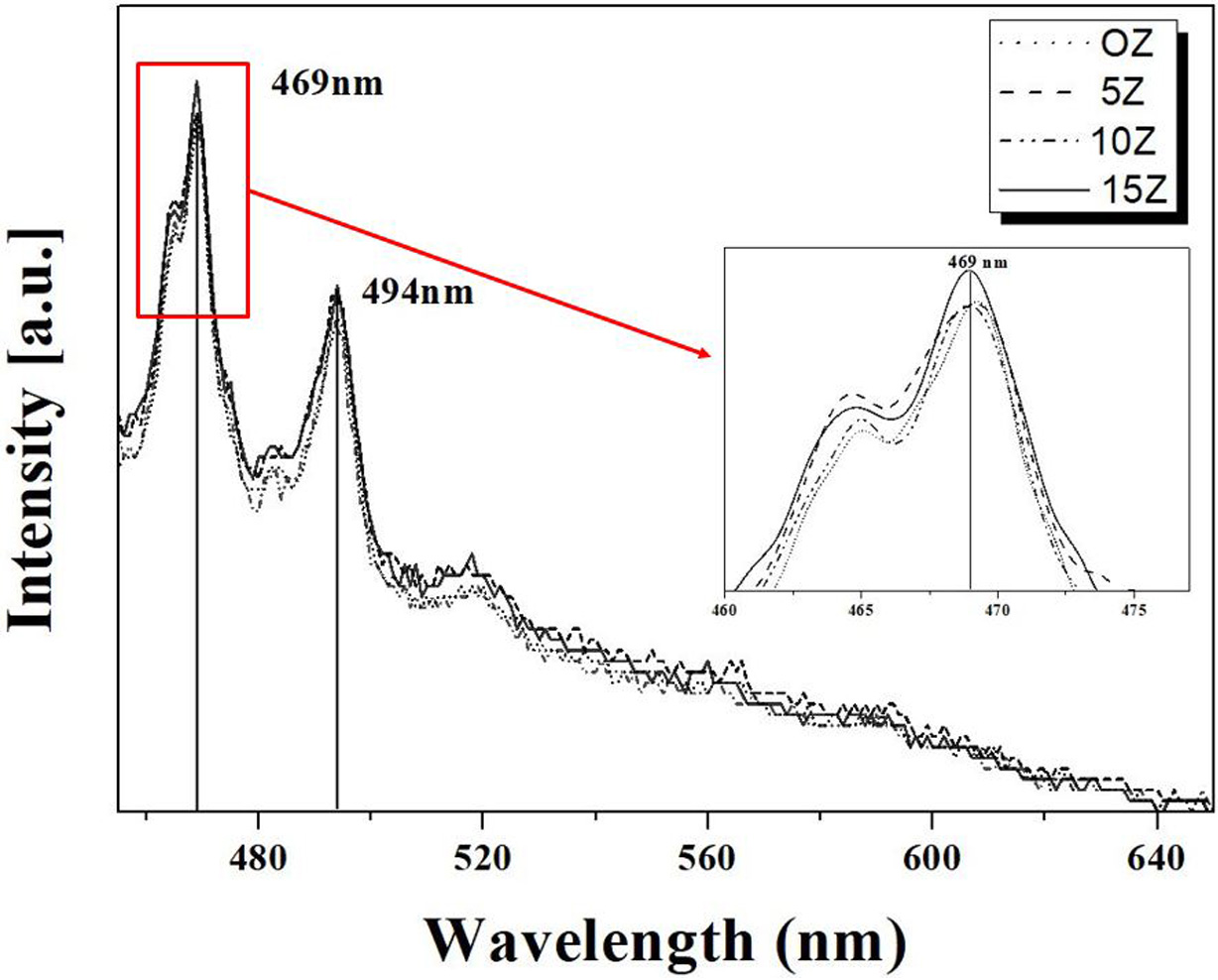
The PL spectra emitted from the glass-ceramics were shown
in Fig. 6 as a function of amount of ZrO2 added. The PL
characteristics of the fresnoite crystal phase are due to the crystal
structure, and when UV light is excited, blue-white light is emitted in the
400-600 nm wavelength range due to the structure of the pyrosilicate
groups (Si2O7) and the square pyramidal groups (TiO2)
[17]. The PL intensities at 469 nm of the 0Z, 5Z and 10Z samples were similar,
but the intensity of the 15Z specimens was the highest among the specimens
prepared in this study. It can be seen that as the amount of
the nucleating agent added increased, the PL intensity increased
overall, and the 15Z specimen showed the best PL properties. As a result, it
can be seen that ZrO2, which acts as a nucleating agent, has a
direct effect on the crystallization of Fresnoite crystals exhibiting PL properties
and has been a factor in improving PL properties.
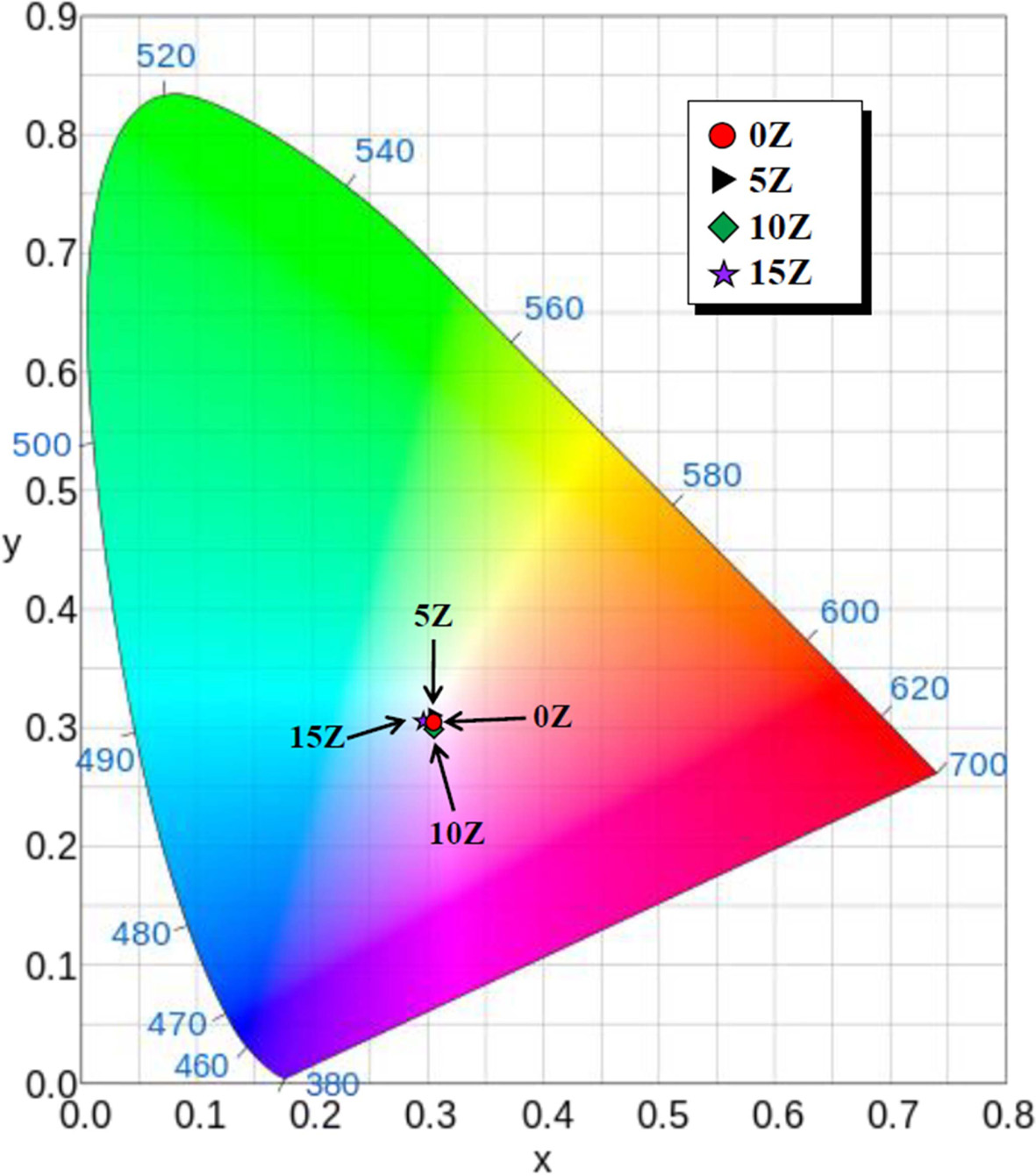
The color of PL spectra of the prepared specimen is
indicated in the CIE color coordinates, as shown in Fig. 7. The PLs of all the
specimens had similar color coordinates, appearing in blue light close to
white.
|
Fig. 1 DTA graph of glass according to amount of ZrO2 added. |
|
Fig. 2 XRD graph of glass-ceramics according to amount of ZrO2 added. |
|
Fig. 3 The cross-section of glass-ceramics observed magnified 200 times with an optical microscope; (a) 0Z and (b) 5Z. |
|
Fig. 4 Microstructure of glass-ceramics according to amount of ZrO2 added observed by SEM. |
|
Fig. 5 PLE spectra of fresnoite-based glass-ceramics. |
|
Fig. 6 PL spectra of fresnoite-based glass-ceramics according to amount of ZrO2 added. The excitation light used has wavelength of 309 nm. |
|
Fig. 7 CIE diagram of light emitted from glass-ceramics with varying amounts of ZrO2 added. |
In this paper, glass-ceramics of BaO : TiO2 : SiO2
= 26 : 13 : 61 (mol%) composition containing 0-15 wt% of ZrO2 as nucleating agent were prepared and its phase identification, microstructure observation and PL analysis were performed. All glass-ceramics showed no or weak crystal peaks, whereas the
strong peaks for fresnoite crystals were formed in the specimen containing 10% ZrO2. The surface
crystallization phenomenon occurred in
0Z and 5Z specimens, meanwhile, in 10Z and 15Z specimens, an internal crystallization occurred. The microstructure of the surface crystal part of the 5Z
specimen was filled with spherical crystals of 80~120 nm in size.
On the other hand, the 15Z specimen showed a
dendrite-shaped crystal phase composed of very small spherical
particles of 10~30 nm in size, indicating that the
amount of ZrO2 added determines the internal or surface
crystallization and the
crystallization rate.
When
the specimens were excited with 309 nm light, all the glass-ceramic specimens emitted 469 nm wavelength light. The
light emission intensity was highest in the 15Z specimen, and the emitted light
showed blue color light close to
white in the Commission International de l'Eclairage (CIE) diagram.
It was possible to produce nano-sized fresnoite-based
glass-ceramics by substituting the nucleating agent ZrO2 into
BaO-TiO2-SiO2 glass having an excellent PL property.
This work was supported by Kyonggi University Research
Grant 2018.
- 1. L. Vladislavova, M. Kracker, T. Zscheckel, C. Thiemeand, and C. Rüssel, Solid State Sci. 78 (2018) 107-115.
-

- 2. G.H. Beall and L.R. Pinckney, J. Am. Ceram. Soc. 82[1] (1999) 5-16.
-

- 3. C.C. Chou, K.C. Feng, I.P. Raevski, H. Chen, C.Y. Tsao, P.Y. Chen, C.S. Chen, C.A. Lu, and C.S. Tu, Mater. Res. Bull. 96 (2017) 66-70.
-

- 4. M. Reben, M. Kosmal, M. Ziąbka, P. Pichniarczyk, and I. Grelowska, J. Non. Cryst. Solids. 425 (2015) 118-123.
-

- 5. L. Wondraczek and P. Pradeau, J. Am. Ceram. Soc. 91 (2008) 1945-1951.
-

- 6. D. A. Duke and G. A. Chase, Applied Optics. 7[5] (1968) 813-818.
-

- 7. A.D. Pablos-Martín, A. Herrmann, C. Patzig, B. Oberleiter, T. Rainer, and Th. Höche, J. Non. Cryst. Solids. 488 (2018) 44-51.
-

- 8. Y. Takahashi, K. Kitamura, Y. Benino, T. Fujiwara, and T. Komatsu, Appl. Phys. Lett. 86 (2005) 1-3.
-

- 9. A. Muller, M. Lorenz, K. Brachwitz, J. Lenzner, K. Mittwoch, W. Skorupa, M. Grundmanna, and T. Hoche, Cryst. Eng. Comm. 13 (2011) 6377-6385.
-

- 10. K. Shinozaki, T. Honma, and T. Komatsu, Mater. Res. Bull. 46[6] (2011) 922-928.
-

- 11. L.L. Martín, P. Haro-González, I.R. Martín, D. Puerto, J. Solís, J.M. Cáceres, and N.E. Capuj, Opt. Mater. 33[2] (2010) 186-190.
-

- 12. H.K. Lee, E.S. Yoo, S.J. Chae, and W.H. Kang, J. Korean Ceram. Soc. 43[9] (2006) 569-574.
-

- 13. S. Liu, J. Wang, J. Ding, H. Hao, L. Zhao, and S. Xia., Ceram. Int. 45[3] (2019) 4003-4008.
-

- 14. W. Wisniewski, K. Thieme, and C. Rüssel, Prog. Mater. Sci. 98 (2018) 68-107.
-

- 15. E. Boulay, C. Ragoen, H. Idrissi, D. Schryvers, and S. Godet, J. Non. Cryst. Solids. 384 (2014) 61-72.
-

- 16. W. Wisniewski, M. Patschger, and C. Rüssel, Sci. Rep. 3[3558] (2013) 1-6.
-

- 17. G. Blasse, J. Inorg. Nucl. Chem. 41[5] (1979) 639-641.
-

 This Article
This Article
-
2020; 21(S1): 58-62
Published on May 31, 2020
- 10.36410/jcpr.2020.21.S1.s58
- Received on Dec 26, 2019
- Revised on Apr 13, 2020
- Accepted on May 4, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seunggu Kang
-
Department of Advanced Materials Science and Engineering, Kyonggi University, Suwon 16227, Korea
Tel : +82-10-5265-2681 - E-mail: sgkang@kgu.ac.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.