- Mechanical properties and electrical resistivity of SiC-TiC composites with nitrate sintering additives
Sung Min Soa,b, Hee Woong Hwanga,c, Sam Heang Yia,c, Joo Seok Parka,*, Kyoung Hun Kima, Kwang Ho Leed, Jongee Parke and Sung Gap Leec
aKorea Institute of Ceramic Engineering and Technology, Jinju, Korea
bInformation Materials Lab. Materials Engineering, Inha University, Incheon, Korea
cCeramic Engineering Department, Gyougsang National University, Jinju, Korea
dBooil Technology co., Ltd., Gunpo, Korea
eDepartment of Metallurgical and Materials Engineering, ATILIM University, Ankara, Turkey
We fabricated SiC-TiC
composites by hot-press sintering with aluminum and yttrium nitrate additive
and evaluated crystal phase, relative density, microstructure, electrical
resistivity and mechanical properties of the sintered body. And the effect of
nitrate additive on the densification of SiC-TiC composites was compared with
that of Al2O3 and Y2O3 additives.
Because nitrate additives were uniformly dispersed in SiC and TiC mixture, it
inhibited the growth of crystal grain between each other and formed fine and
uniform microstructure, thereby improving mechanical properties and electrical
resistivity. The electrical resistivity and flexural strength of the SiC-TiC
composite with aluminum and yttrium nitrate additive were 2.3 Ω·cm and 652.3
MPa respectively.
Keywords: SiC-TiC, Composites, Mechanical properties, Electrical resistivity, Nitrate additive
Silicon carbide (SiC) forms tetrahedron basic structure
by sp3 hybrid orbital function of carbon (C) and silicon (Si), and
strong covalent non-oxide having σ-bond between C and
Si. It is a ceramic material with excellent mechanical
properties such as hardness, strength, elastic modulus, and
abrasion resistance compared to other ceramic materials and additionally has
high thermal conductivity and stable properties to various acids and bases
[1-5]. As a result, it is applied as a core component in
various industries such as abrasives, heating elements, high
temperature structural materials, heat exchangers, mechanical
seals, bulletproof materials, and semiconductor manufacturing
process parts [6-9]. SiC can be classified into β-SiC
of cubic structure and α-SiC of a hexagonal and rhombohedral structure
according to the crystal structure. The α-SiC has different polytypes according
to the stacking sequences such as 4H, 6H, 15R, etc., and it is known that
various physical and electrical properties are expressed by this crystal
structure [10]. High purity SiC has a low electrical conductivity close to the
insulator, but recently, there has been a growing interest in
the application of SiC materials to the devices used
in extreme environments such as high temperature, high power,
and high frequency by controlling electric conductivity or an electrodischarge
machining for the processing of semiconductor process equipment parts or
complex shaped parts that require various electrical conductivity [11-13].
The electrical resistance of SiC can be lowered by doping
nitrogen (N) atoms in the SiC lattice, sintering with liquid phase additives
such as Al2O3, Y2O3, Sc2O3,
etc. in nitrogen atmosphere, or adding conductive additives such as TiN, ZrN,
TiC, TiB2 which helps to improve electrical conductivity [14-16].
Among them, TiC has the highest hardness, modulus of elasticity, coefficient of
thermal expansion, and lower electrical resistance
than SiC. Therefore, when forming a composite with SiC it
is expected to lower the electrical resistance and to improve mechanical
properties compared to SiC monoliths through particle strengthening effect
[17]. SiC-TiC composites can be prepared through liquid phase sintering with
the addition of oxide sintering aids such as Y2O3,
Al2O3-Y2O3, or reaction sintering
through a reduction of TiO2 added in SiC [18, 19]. In
addition, pressure sintering methods such as hot press sintering and spark
plasma sintering are used to manufacture products that are dense and have
excellent mechanical properties [20].
The study of SiC-TiC composites has been mainly focused
on improving mechanical properties such as fracture toughness through particle
strengthening mechanism. It is known that residual
stress due to mismatch of thermal expansion coefficient of TiC (α =
7.4 × 10-6 K-1)
particles uniformly dispersed in the SiC matrix (α = 4.5 × 10-6 K-1)
suppress the progress of cracks, thereby increasing the fracture toughness and
strength. Furthermore, Wei and Becher reported that the fracture toughness
increased by 50% when the TiC content in the SiC-TiC composite was 24.6 vol%
[21-24]. On the other hand, researches on the electrical properties of SiC-TiC
composites are rare. M. Khodaei et al. prepared a SiC-TiC composite
showing an electrical resistance of 2.2 × 105
Ω·m by the reaction between TiO2 and SiC [25].
Therefore, this study aims to manufacture low-resistance SiC-TiC
composites with excellent mechanical properties using Y3Al5O12
(yttrium aluminum garnet, YAG) as a liquid sintering aid. The electrical
resistance of SiC-TiC composites is greatly influenced by the grain size of the
components constituting the matrix, the amounts of additives,
and the uniform dispersion [26]. Therefore, aluminum
nitrate nonahydrate (Al(NO3)3·9H2O) and
yttrium nitrate hexahydrate Y(NO3)3·6H2O
dissolved in ethanol are used as sintering aids to uniformly control the
microstructure, and accordingly, the electrical resistance and mechanical
properties were investigated.
α-SiC powder (0.5 μm, 15C, Saint-Gobain, Norway) and TiC
powder (2-3 μm, Pacific Particulate Materials, Canada) with 6H crystal
structure were used as starting materials to prepare SiC-TiC
composites. Al(NO3)3·9H2O (98%,
Sigma-Aldrich, USA) and Y(NO3)3·6H2O (99.8%,
Sigma-Aldrich, USA) were used as sintering aids. Here, Al2O3
(0.2 μm Sumitomo Chemical, Japan) and Y2O3 (2 μm, high
Purity Chemical, Japan) were used as oxide sintering aids for comparison with
nitrate sintering aids. The raw materials were weighted as shown in Table 1 and
mixed mechanically for 12 h with SiC balls in a polypropylene jar using ethanol
medium. The mixed slurry was dried and passed through
200 mesh sieve to prepare a SiC-TiC mixed powder. The prepared powder was
placed in a graphite mold of 50 × 50 mm, and then sintered at
1,800-1,950 oC for 1 h under 40 MPa pressure in a nitrogen
atmosphere using a hot-press sintering furnace (SHP-30; Samyang Ceratech, South
Korea) to form a SiC-TiC composite.
The density of the prepared SiC-TiC composites was
measured using the Archimedes method, and the crystal phase analysis was
performed using an X-ray diffractometer (Smart Lab, Rigaku, Japan). The SiC-TiC
sintered body was tailored by 3×4×35 mm, and then the bending strength was
measured by 3 point method under span 30 mm and head speed 0.5 mm/s according
to ISO 14704 standard. Fracture toughness was calculated by measuring the
indented crack length using the formula (KIC =
0.018 (E/H) 0.5(P/C1.5) proposed by Miyoshi
et al. [27]. E is the modulus of elasticity, H is the Vickers hardness, P is
the indentation load, and C is half the average crack length. The
microstructure of the sintered body was observed using FE-SEM (JSM-7100F; Jeol,
Japan), and thermal conductivity was calculated by the equation
((k = ρ·Cp·D (k: thermal conductivity, ρ: density, Cp: heat capacity,
D: thermal diffusion
coefficient)) after measuring the thermal diffusion coefficient using a laser flash device (LFA 427, NETZSCH, Germany). The
specific resistance of the SiC-TiC
composites was measured using a resistance measuring instrument (CMT-SR1000N, Advanced Instrument Technology,
USA).
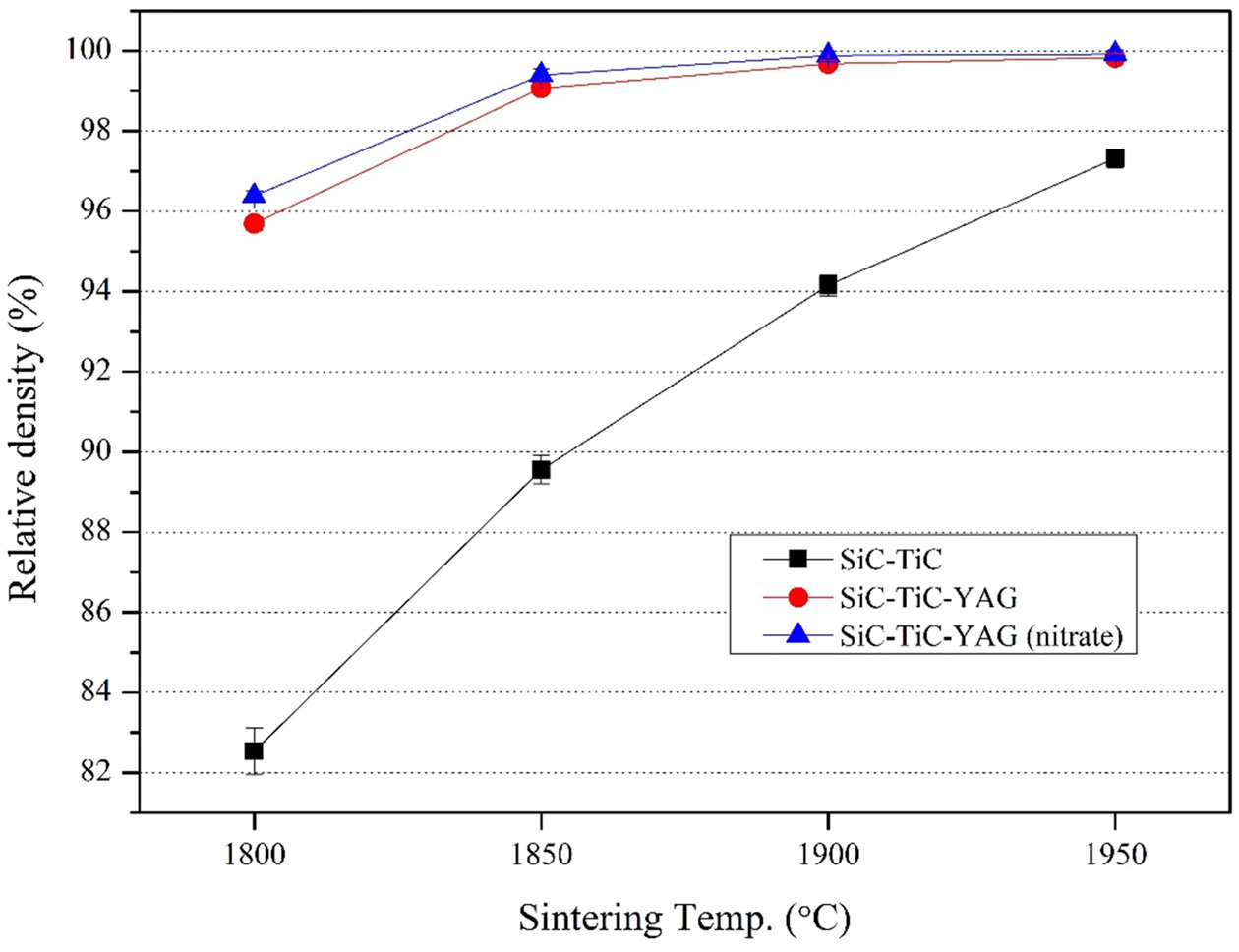
Fig. 1 is a graph showing the relative density of the
composite according to the sintering temperature. The relative density
increased with increasing sintering temperature. Reaching a maximum value at
1,950 oC. ST (SiC-30 vol% TiC) composition without sintering
aid shows a low relative density of about 82.5-97.3% depending on the sintering
temperature. However, STY (SiC-30 vol% TiC-2 vol% YAG) and STYN (SiC-30 vol%
TiC-2 vol% YAG) compositions with the sintering aid show the
relative density of 99% or more at the sintering temperature of 1,850 oC
or higher. Comparing the relative densities of nitrate and oxide sintering
aids, the relative density of STYN was slightly higher than that of STY from
the sintering temperature of 1,800 °C and the relative density of STY was
about 99.1% at 1,850 oC while that of STYN was 99.4%. Through
this result, it was found that nitrate sintering aid acts at a lower
temperature than oxide sintering aid, promotes sintering, and helps to
manufacture a denser sintered body [26].
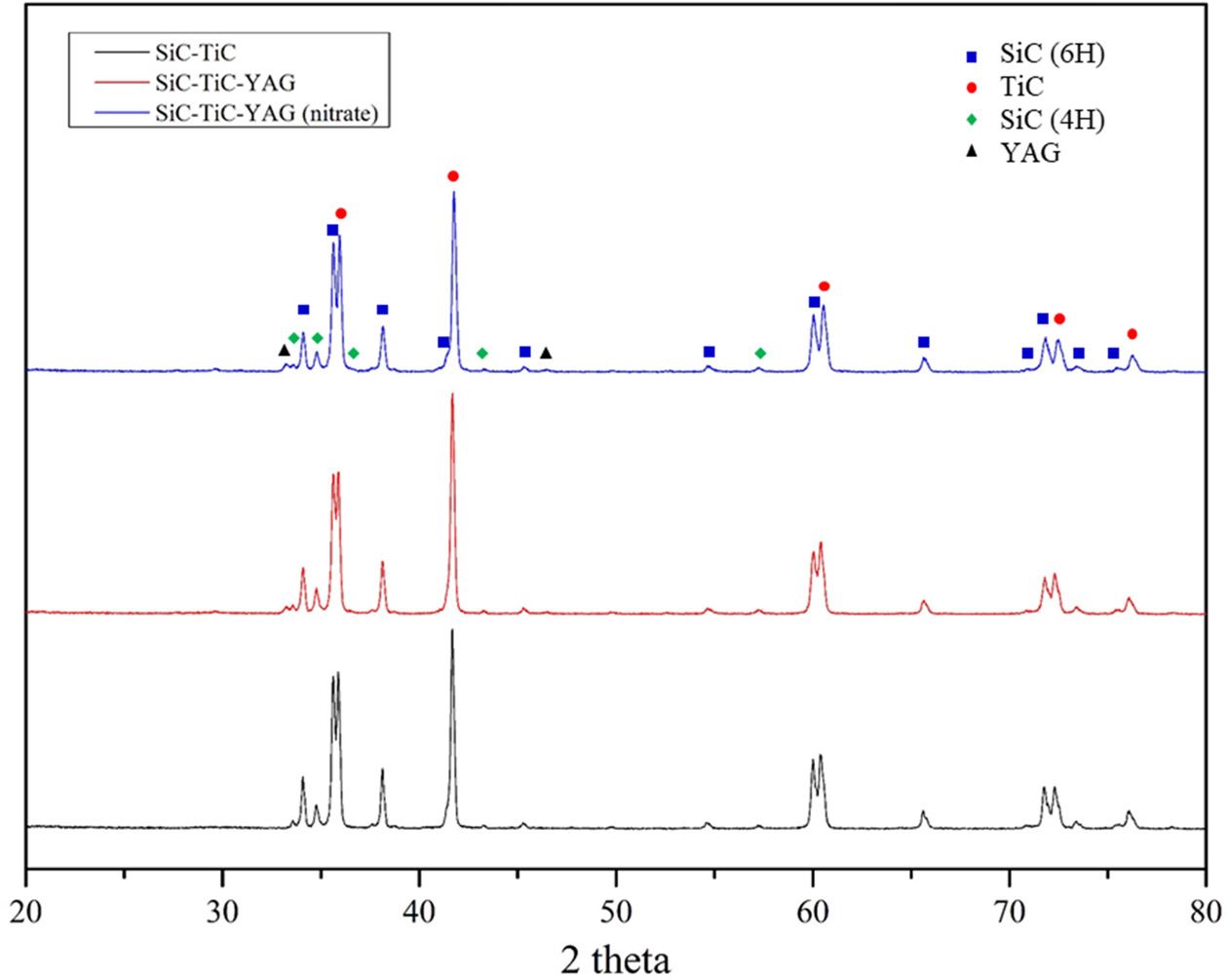
Fig. 2 is the result of crystal phase analysis obtained
through x-ray diffraction analysis. In all specimens, the main crystalline
phases were analyzed by 6H-SiC and TiC, and 4H crystalline phases not included
in the starting material were observed, indicating that SiC was phase-shifted
from 6H to the 4H structure. This phase transition is thought to be caused by
TiC because it occurs even in the composition without the sintering aid. In the
compositions to which the sintering aid was added, the YAG crystal phase was
observed from 1,800 °C, and it was confirmed that the liquid phase was formed
at a low temperature and involved in densification.
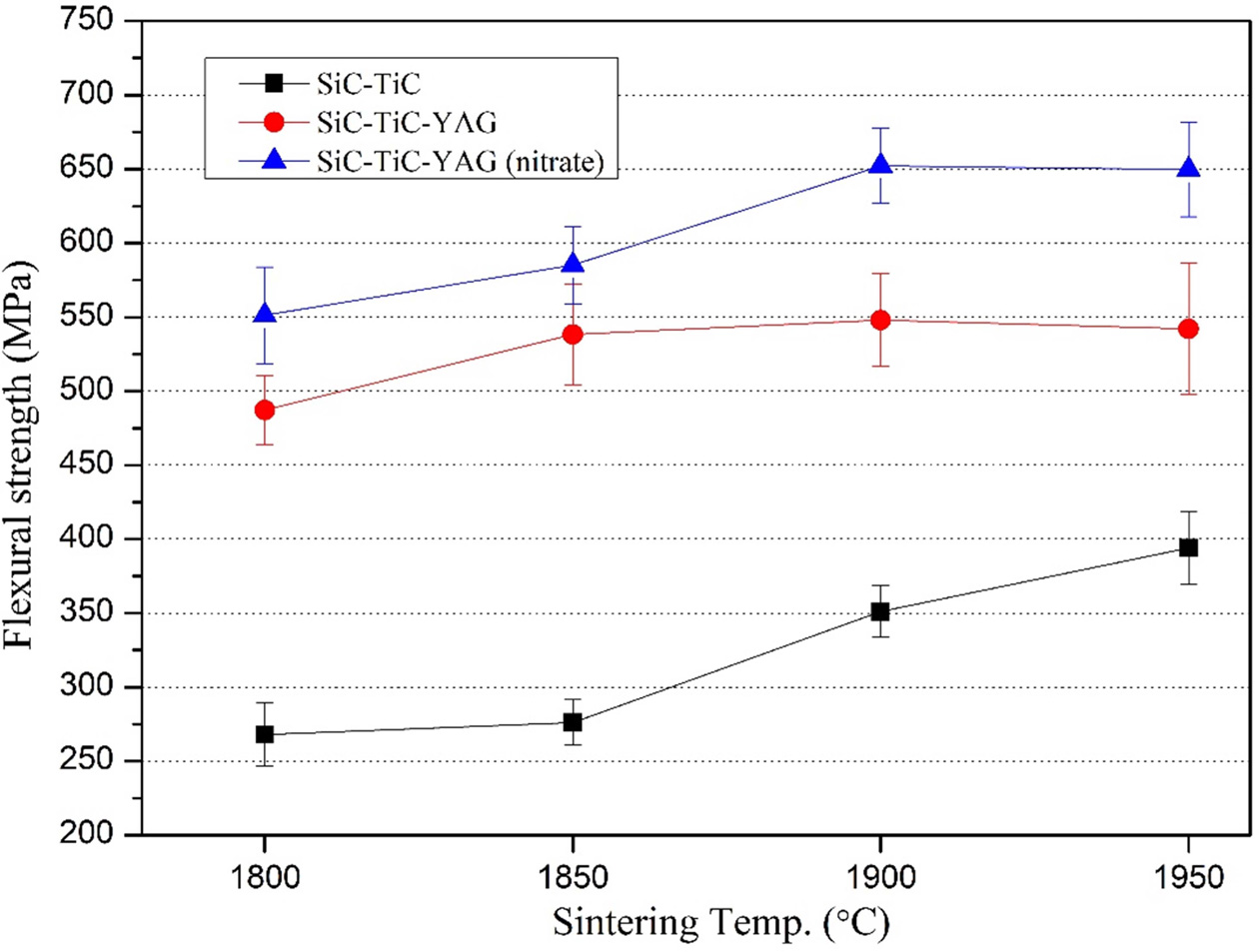
The bending strength results of SiC-TiC composites were
shown in Fig. 3. As the sintering temperature increases, the bending strength
tends to increase, and when the sintering aid is not added, the relative
density is low and the porosity is high, resulting in low strength. However,
the composition with the sintering aid showed a high bending strength due to
the increase of the relative density induced by rapid densification and showed
a maximum value at 1,900 oC. Therefore, it can be seen that the
bending strength of STYN composition is better because the bending strength of STY
is 547.9 MPa while the bending strength of STYN is
652.3 MPa. Also, as the sintering temperature increased from
1,900 oC to 1,950 oC, the bending strength
decreased slightly. This is thought to be due to the grain growth
at 1,950 oC sintering temperature, as can be seen from Fig. 3
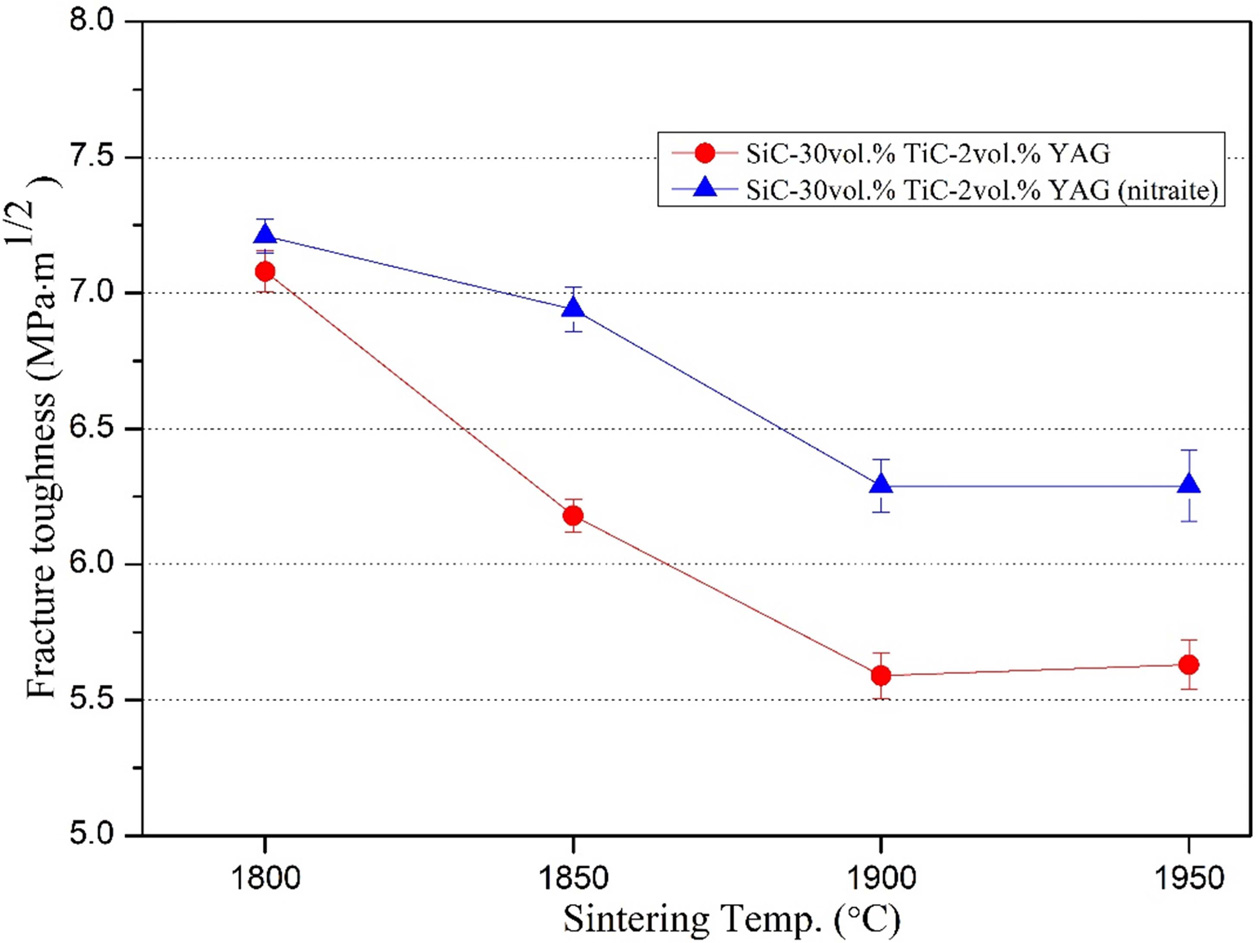
and 4. The reason why the bending strength is relatively high in the case of
using the sintering aid of nitrate is thought to be that the fracture behavior is
different as well as the influence of the high relative density. As seen in
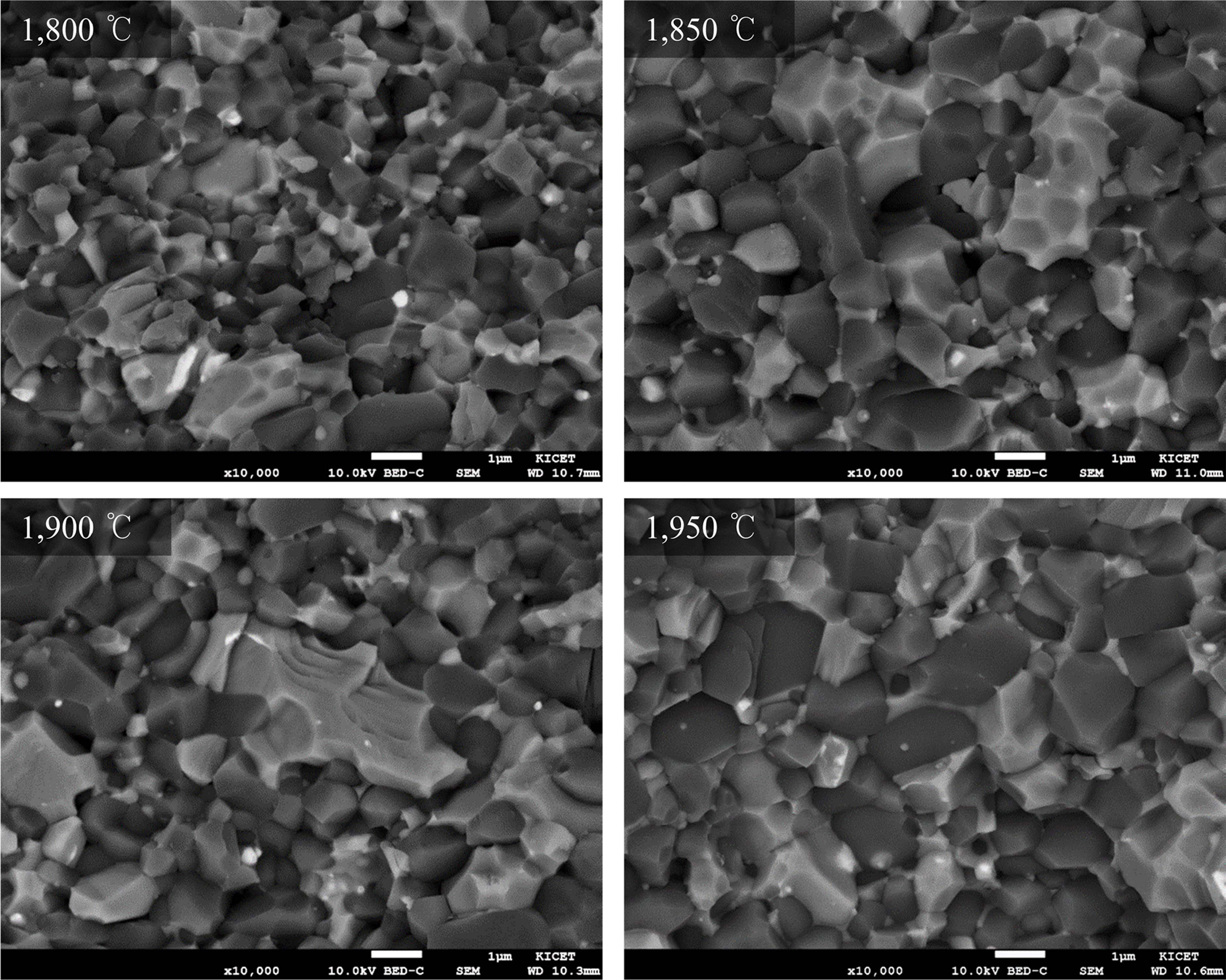
Fig. 3 and 4 of fractured surface FE-SEM
images, the fracture shape of STY specimens shows more intragranular intergranular while that of STYN
specimens shows both intragranular and intergranular. As such, the intergranular fracture in SiC-TiC composites is due to the
strong interfacial bonds between the grains, and both the strength and fracture
toughness are enhanced because more energy is consumed when cracks propagated through the grains of the composites.
The addition of nitrate sintering aids showed that the YAG crystal size is smaller and more uniformly distributed in the
microstructure than the oxide sintering aids, and as a result, strong
interfacial bonds are formed between grains of the composite [28, 29].
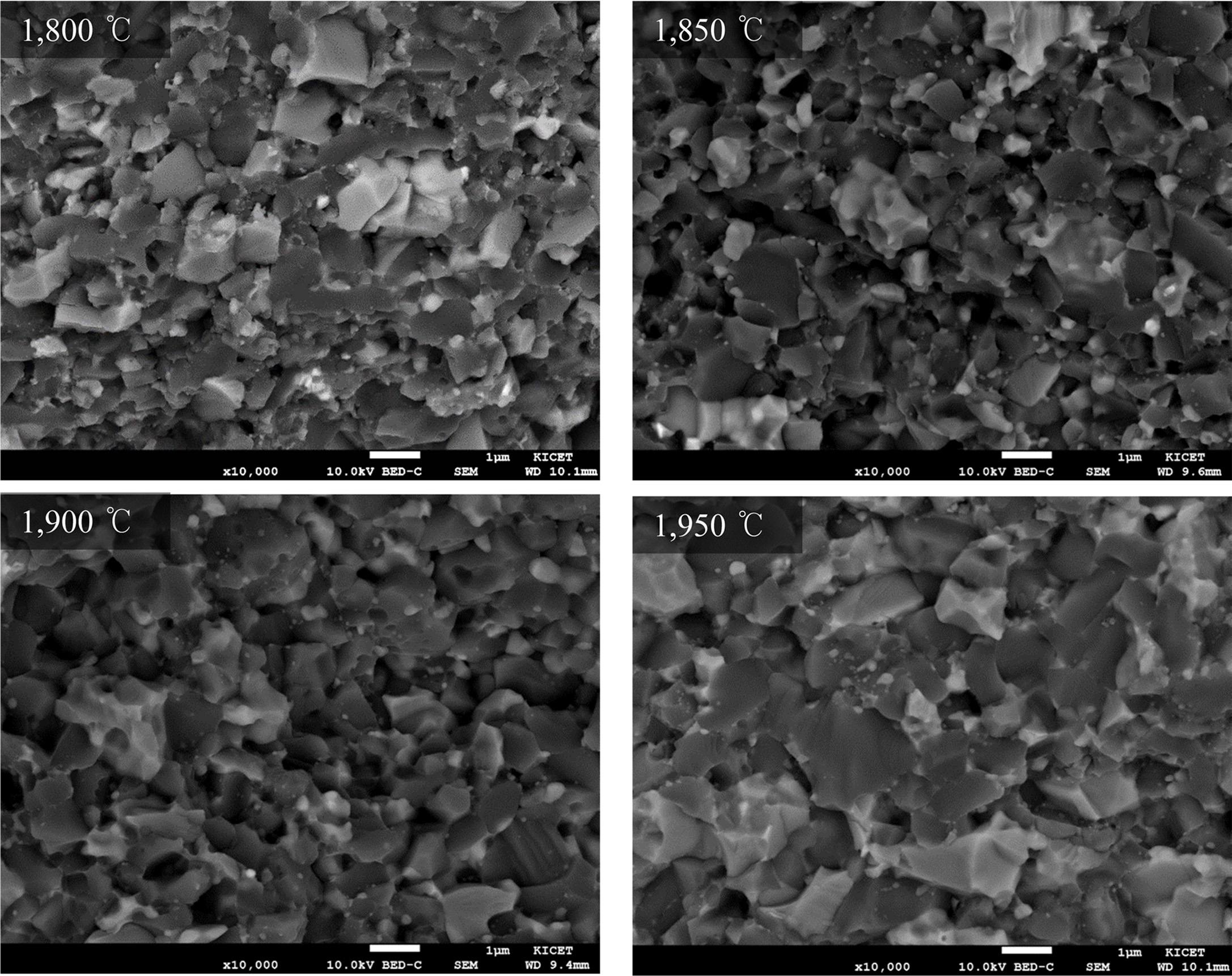
Fig. 6 shows the Vickers hardness results of SiC-TiC
composites according to the sintering temperature. The hardness tends to
increase with increasing the sintering temperature,
which is due to the increase in the sintering density and
similar to the relative density graph (Fig. 1). At 1,900 oC,
the hardness of STY composition was 22.55 GPa and STYN composition was 52.14
GPa, which showed better hardness when nitrate sintering aid was added. The
hardness decreases as the sintering temperature increases to 1,950 oC,
which may be due to grain growth as can be seen in the microstructure photographs
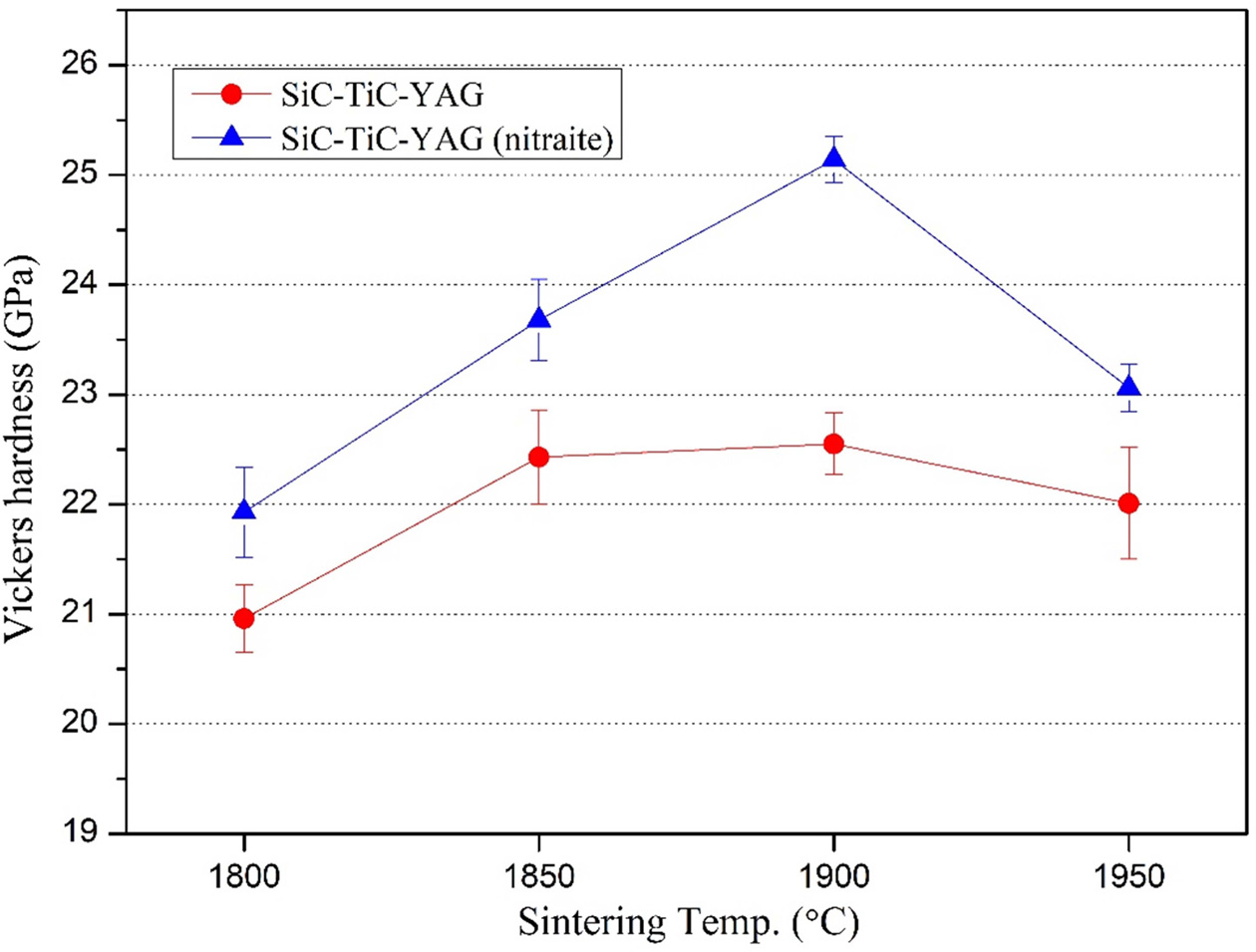
(Fig. 4 and 5). The fracture toughness of composites with sintering temperature
is shown in Fig. 7. Higher fracture toughness results in STYN composition with
a nitrate sintering aid. Fracture toughness in grain-reinforced
ceramic composites is known to be enhanced by residual stresses in thermal
expansion coefficients and elastic modulus differences between the matrix and
dispersed ceramic particles. The fracture toughness is enhanced by the residual
stress on the matrix when the coefficient of thermal expansion of the added
particles is greater than that of the matrix. Therefore, in the SiC-TiC
composites, the thermal expansion coefficients of SiC and TiC are 4.5 × 10-6 K-1
and 7.4 × 10-6 K-1,
respectively, so that compressive residual stress is formed
on the SiC because of the smaller coefficient, and the
toughness is enhanced compared to monolithic SiC [30, 31].
To understand the fracture mode and the strengthening
mechanism of the SiC-TiC composite in more detail, the cracks induced by
Vickers indenters were observed by FE-SEM and shown in Fig. 8. It was confirmed
that crack toughening and branching occurred in all compositions containing
nitrate and oxide sintering aids, which prevented crack propagation and
enhanced fracture toughness [32].
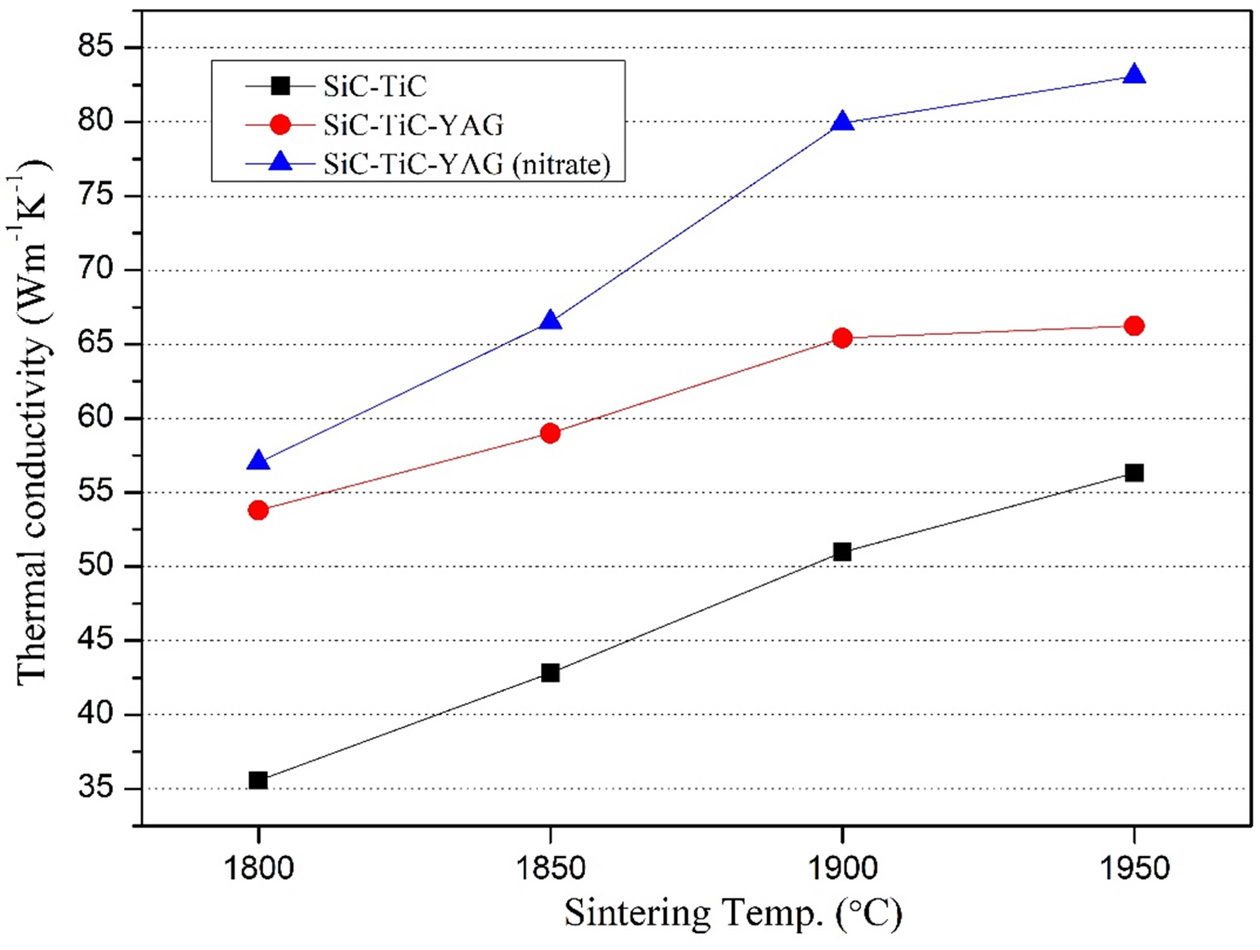
Fig. 9 is the thermal conductivity measurement result of
the SiC-TiC composite. As the sintering temperature increased and densification
proceeded, the thermal conductivity increased. However, the
composite without sintering aid showed low thermal conductivity due
to low density and low porosity inside the specimen.
STYN sample showed the best thermal conductivity as 83
W/mK at sintering temperature of 1,950 oC. Fig. 10 shows the
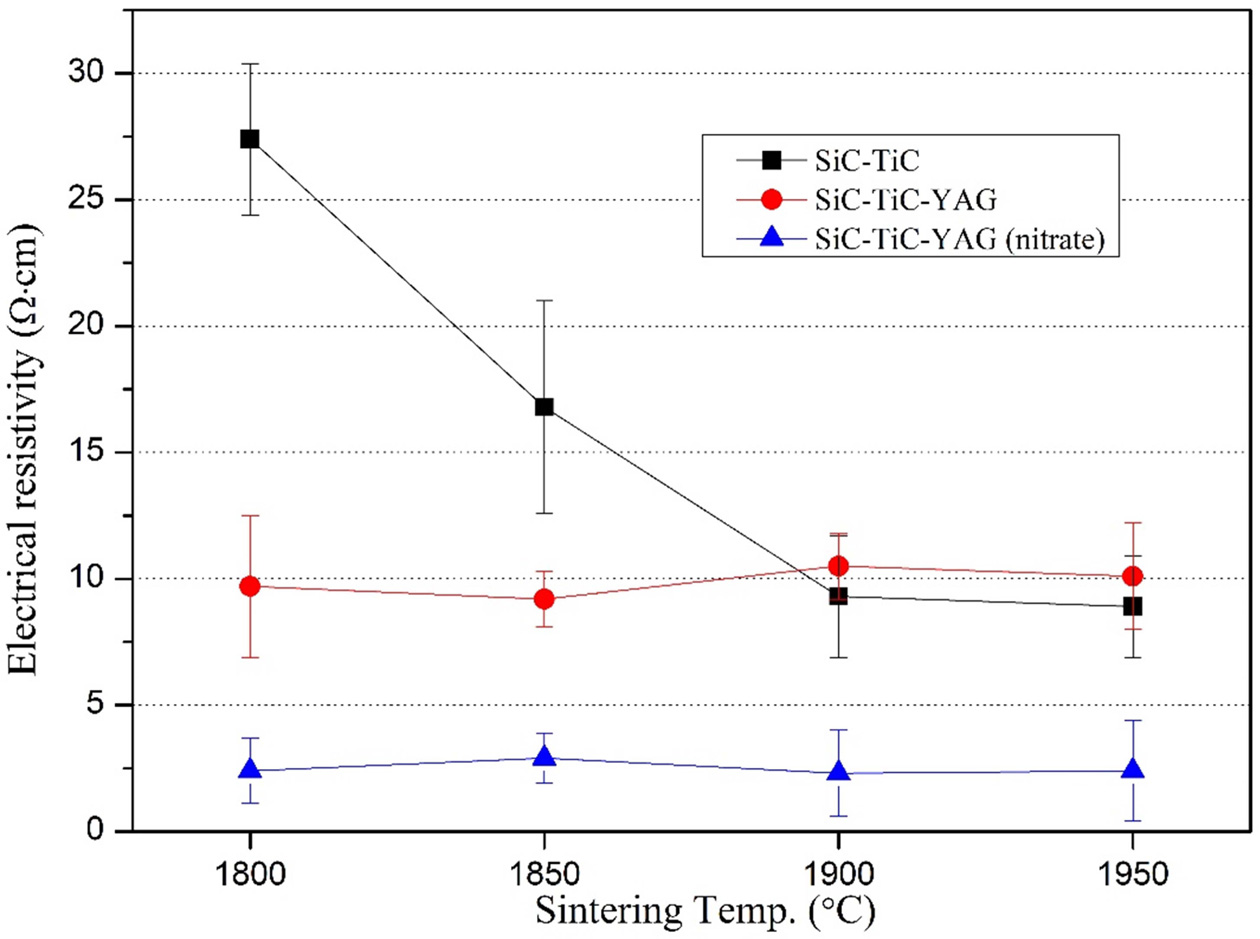
electrical resistance of the SiC-TiC composite according to the sintering temperature.
In the composition without the sintering aid, the electrical resistance
decreased significantly as the densification progressed. When sintering was
performed at 1,900 and 1,950 oC, the value was lower than that
of the STY composition containing the oxide sintering aid. This may
be because YAG, which has high electrical resistance, is present
at the grain boundaries and triple points of SiC and TiC, thereby lowering the
overall electrical conductivity. On the other hand, in the composition
containing nitrate sintering aid, the YAG phase was found to have the lowest
electrical resistance, because it exists finely and uniformly compared to the
oxide composition, and it was found to have a low electrical resistance of 2.3 Ω·cm
at 1,900 oC.
|
Fig. 1 Variation of relative density of SiC-TiC composites with the sintering temperature. |
|
Fig. 2 XRD patterns of SiC-TiC sintered specimens at 1,950?. |
|
Fig. 3 Variation of flexural strength of SiC-TiC composites with Fig. 2. XRD patterns of SiC-TiC sintered specimens at 1,950?. the sintering temperature. |
|
Fig. 4 Fracture surfaces of SiC-TiC-YAG composite. |
|
Fig. 5 Fracture surfaces of SiC-TiC-YAG (nitrate) composite. |
|
Fig. 6 Variation of Vickers hardness of SiC-TiC composites with the sintering temperature. |
|
Fig. 7 Variation of fracture toughness of SiC-TiC composites with the sintering temperature. |
|
Fig. 8 SEM image of the crack paths of SiC-TiC composites sintered at 1,900 oC. |
|
Fig. 9 Variation of thermal conductivity of SiC-TiC composites with the sintering temperature. |
|
Fig. 10 Variation of electrical resistivity of SiC-TiC composites with the sintering temperature. |
In this study, compact and uniform SiC-30 vol% TiC
composites having a relative density of about 99.9% at 1,900 oC
could be prepared by hot press sintering method adding aluminum nitrate and
yttrium nitrate as sintering aids. Nitrate sintering aid is dissolved in
ethanol and can be uniformly mixed with the raw material, which promotes
densification of SiC-TiC composite at a lower temperature than oxide sintering
aid, and forms a uniform and fine YAG phase to improve mechanical properties of
the sintered body. With sintering at 1,900 oC, the bending
strength was 652.3 MPa, the Vickers hardness was 25.14 GPa, and the fracture
toughness was 6.29 MPa·m1/2. Since the electrical conductivity of
SiC-TiC ceramic composites is greatly influenced by the grain
size of the components constituting the matrix and the
amounts of additives and uniform dispersion, SiC-TiC composites with the
electrical resistance of 2.3 Ω·cm could be prepared at 1,900 oC
sintering temperature with the addition of nitrate.
This work was supported by the Technology Innovation Program
(10067558, Development of functional watches with ceramic
composite) funded by the Ministry of Trade, Industry & Energy (MOTIE,
Korea)
- 1. Y.G. Gogotsi, P. Kofstad, M. Yoshimura, and K.G. Nickel, Diam. Relat. Mater. 5[2] (1996) 151-162.
-

- 2. A. Noviyanto and D. Yoon, Curr. Appl. Phys. 13[1] (2013) 287-292.
-

- 3. O. Lopes, A. Ortiz, F. Guibertuau, and N. Padture, J. Eur. Ceram. Soc. 27[11] (2007) 3351-3357.
-

- 4. S. Prochazka and R. M. Scanlan, J. Am. Ceram. Soc. 58[12] (1975) 72-72.
-

- 5. R.A. Alliegro, L.B. Coffin, and J.R. Tinklepsugh, J. Am. Ceram. Soc. 39[11] (1956) 386-389.
-

- 6. D. Sciti and A. Bellosi, J. Mater. Sci. 35[15] (2000) 3849-3855.
-

- 7. K. Biswas, G. Rixecker, and F. Aldinger, J. Eur. Ceram. Soc. 23[7] (2003) 1099-1104.
-

- 8. M. Balog, P. Šajgalı́k, M. Hnatko, Z. Lenčéš, F. Monteverde, J. Kečkéš, and J.-L. Huang, J. Eur. Ceram. Soc. 25[4] (2005) 529-534.
-

- 9. S. Kaur, R. Riedel, and E. Ionescu, J. Eur. Ceram. Soc. 34[15] (2014) 3571-3578.
-

- 10. H. Harima, Microelectron. Eng. 83[1] (2006) 126-129.
-

- 11. K.J. Kim, S.H. Jang, Y.W. Kim, B.K. Jang, and T. Nishimura, Ceram. Int. 42[15] (2016) 17892-17896.
-

- 12. J.R. Jenny, D.P. Malta, St G. Müller, A.R. Powell, V.F. Tsvetkov, H. McD Hobgood, R. C. Glass, and C. H. Carter Jr., J. Electron. Mater. 32[5] (2003) 432-436.
-

- 13. F. Siegelin, H.J. Kleebe, and L.S. Sigl, J. Mater. Res. 18[11] (2003) 2608-2617.
-

- 14. K.J. Kim, J.H. Eom, Y.W. Kim, W.S. Seo, M.J. Lee, and S.S. Hwang, Ceram. Int. 43[15] (2017) 5343-5346.
-

- 15. G. Augustine, V. Balakrishna, and C.D. Brandt, J. Cryst. Growth 211[1-4] (2000) 339-342.
-

- 16. T.Y. Cho, Y.W. Kim, and K.J. Kim, J. Eur. Ceram. Soc. 36[11] (2016) 2659-2665.
-

- 17. J. Cabrero, F. Audubert, and R. Pailler, J. Eur. Ceram. Soc. 31[3] (2011) 313-320.
-

- 18. M. Khodaei, O. Yaghobizadeh, N. Ehsani, H.R. Baharvandi, and A. Dashti, Ceram. Int. 44[14] (2018) 16535-16542.
-

- 19. O. Agac, M. Gozutok, H.T. Sasmazel, A. Ozturk, and J. Park, Ceram. Int. 43 (2017) 10434-10441.
-

- 20. H. Endo, M. Ueki, and H. Kubo, J. Mater. Sci. 26 (1991) 3769-3774.
-

- 21. D. Ahmoye, D. Bucevac, and V.D. Krstic, Ceram. Int. 44[12] (2018) 14401-14407.
-

- 22. D. Shaoming, J. Dongliang, T. Shouhong and G. Jingkun, J. Mater. Sci. Lett. 15 (1996) 394-396.
-

- 23. H.G. An, Y.W. Kim and J.G. Lee, J. Eur. Ceram. Soc. 21[1] (2001) 93-98.
-

- 24. G.C. Wei and P.F. Becher, J. Am. Ceram. Soc. 67[8] (1984) 571-574.
-

- 25. M. Khodaei, O. Yaghobizadeh, N. Ehsani, and H.R. Baharvandi, Int. J. Refract. Met. Hard Mater. 76 (2018) 141-148.
-

- 26. J.Y. Kim, T. Iseki, and T. Yano, J. Am. Ceram. Soc. 79[10] (1996) 2744-2746.
-

- 27. T. Miyoshi. N. Sagawa, and T. Sasa, J. Jpn. SpC. Mech. Eng. A 51[471] (1985) 2489-2497.
-

- 28. Z. Zhang, X. Du, W. Wang, Z. Fu, and H. Wang, Int. J. Refract. Met. Hard Mater. 41 (2013) 270-275.
-

- 29. Y. Azizian-Kalandaragh, A. Sabahi Namini, Z. Ahmadi, and M. Shahedi Asl, Ceram. Int. 44[16] (2018) 19932-19938.
-

- 30. M. Taya, S. Hayashi, A.S. Kobayashi, and H.S. Yoon, J. Am. Ceram. Soc. 73[5] (1990) 1382-1391.
-

- 31. S.M. So, W.H. Choi, K.H. Kim, J.S. Park, M.S. Kim, J. Park, Y.S. Lim, and H.S. Kim, Ceram. Int. 46[7] (2020) 9575-9581.
-

- 32. M.S. Asl, Z. Ahmadi, A.S. Namini, A. Babapoor, and A. Motallebzadeh, Ceram. Int. 45[16] (2019) 19808-19821.
-

 This Article
This Article
-
2020; 21(S1): 16-22
Published on May 31, 2020
- 10.36410/jcpr.2020.21.S1.s16
- Received on Dec 17, 2019
- Revised on Mar 22, 2020
- Accepted on Mar 24, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Joo Seok Park
-
Korea Institute of Ceramic Engineering and Technology, Jinju, Korea
Tel : +82-55-792-2775
Fax: +82-55-792-2796 - E-mail: pjuju@kicet.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.