- Systematic experimental study for optimized hydrogen production of Ni/CeO2 prepared by incipient wetness impregnation technique
Shian Lia, Qiuwan Shena,* and Bengt Sundenb
aMarine Engineering College, Dalian Maritime University, Dalian, China
bDepartment of Energy Sciences, Lund University, Lund, Sweden
In this present work,
different supports (CeO2/ZrO2) and different Ni contents
based catalysts were prepared by incipient wetness impregnation method and
tested in the methane steam reforming (MSR) reaction. The prepared catalysts
were characterized by X-ray diffraction (XRD) and scanning electron microscope
(SEM). The XRD analysis confirmed the prepared Ni/CeO2 samples have
a pure crystal structure. In the catalytic tests, the effects of reactivity
temperature, different support, Ni loading content and the gas hourly space
velocity (GHSV) on hydrogen production performance were investigated
systematically. The results showed that the optimal reaction conditions for MSR
by Ni/CeO2 catalyst were as follows: 10 wt % Ni/CeO2,
750 oC, 1500 h-1. The stability test for 12 h
at 750 oC indicated that the chosen Ni/CeO2 catalyst
had excellent thermal stability.
Keywords: Hydrogen, Steam reforming, Ni, CeO2, Support
Nowadays, hydrogen is becoming an environmental friendly
energy source producing clean energy in an efficient way. Compared with new
energy sources such as solar energy and wind energy, hydrogen is a
secondary energy source that can be produced in a wide variety of
ways, such as photolysis of water, electrolysis of water, and hydrocarbon
reforming. The most mature hydrogen production technology is hydrocarbon
reforming. Com- pared
with hydrolyzed hydrogen production, hydrocarbon reforming
has the characteristics of low cost and high hydrogen production rate.
Methane is widely found in natural gas and biogas in
nature. Methane reforming is a widely used method in industries to convert
natural gas to H2 or syngas (H2+CO) [1-5]. According to
different reforming raw materials, it can be divided into partial oxidation of
methane [6], carbon dioxide reforming of methane [7, 8] and steam
reforming of methane [9, 10]. The methane steam
reforming reaction (MSR) is an endothermic reaction and is a fairly mature
industrial hydrogen production technology.
The reaction is represented by Eq. (1).
CH4 + H2O →
CO + 3H2 ΔH = +206.29
KJ/mol (1)
CO + H2O → CO2 + H2 ΔH = -41.19 KJ/mol (2)
This main reaction occurs at a high temperature, followed
by a water gas shift reaction (WGS) which produces CO2 (eq. (2)).
The methane steam reforming reaction is a complicated
process, which may involve multiple reactions. The reaction flows from the
surface of the catalyst by water vapor and methane under a high
temperature atmosphere. After the catalyst is catalyzed, H2
and CO are formed. CO2 will be generated when water is excessive.
The reaction is carried out under a certain pressure and high temperature.
In such a harsh environment, carbon deposition may occur.
Commonly used noble metal catalysts are Pt, Pd, and Rh.
The precious metal catalyst has a certain anti-carbon deposition performance,
and has better catalytic performance and stability than the general catalyst.
However, it is not widely used due to the expensive price.
It has been reported that noble metal catalysts such as
Rh, Ru, Pd and Pt have high activity, stability and carbon deposition
resistance [11-13]. Among many traditional metal catalysts, Ni-based catalysts
stand out due to their lower cost,good catalytic and higher stability
performance at high temperatures [14-16].
The support is one of the essential components of the
catalyst. It plays a very important role in the catalyst. One of its basic
functions is to support the active component.
The support determines the catalytic property of the system. For some specific reactions, besides affecting metal
dispersion and providing stability for metal particles, the support can also participate
in the reactions [17, 18]. Commonly used supports are γ-Al2O3,
CeO2, ZrO2, SiO2 and others. The support is
required to have high mechanical strength, large specific surface area and
strong anti-sintering ability. Due to its unique properties, cerium has been
widely used in heterogeneous catalysis [19].
Several studies have applied these materials for MSR and
concluded that the intermediate metal loadings produce the
best catalytic performance [20-22]. A series of nickel
catalysts with different metallic contents supported on CeO2 and ZrO2
were studied by Dong et al. [20]. They found that nickel loading of 15% wt is
optimal. Because it balances the two different catalytic activation points of
CH4 and H2O. In addition, Roh et al. studied the effect
of nickel content on a Ce-ZrO2/Al2O3 support
in MSR, and their results showed that with the nickel content of 12% wt.,
methane has the maximum conversion [21].
In the present study,
different supports (CeO2/ZrO2) and different Ni contents
are used to optimize their composition as catalyst for hydrogen production from
the MSR reaction. A series of synthesized catalysts were thoroughly
characterized by XRD and SEM. In addition, in order to evaluate the optimal
conditions of this process, the influence of the reactivity temperature,
different supports, Ni content and the gas hourly space velocity (GHSV) were
investigated to achieve a deeper understanding of Ni-based supported catalysts.
Samples
preparation
To prepare a catalyst using a wet incipient wetness
impregnation method, it is first necessary to measure the water absorption of
the support. Weigh a certain mass of the support in the beaker which is defined
as a, return the analytical balance to zero, add deionized water
to the fully immersed carrier with a plastic dropper, measure the
mass of deionized water to b, seal the beaker mouth at room temperature After
standing for 12 hours, the deionized water not absorbed by the carrier was
filtered off, and the mass was found to be m. Then the support water absorption
rate X is:
X = (b - m)/a*100% (1)
Preparation of different wt% Ni loadings catalysts
Ni(NO)3·6H2O having a corresponding
Ni loading amount was weighed into a beaker, and CeO2 was used as a
support. Deionized water was added to Ni(NO)3·6H2O according to the
measured water absorption rate of the support
to prepare a precursor solution. After the Ni(NO)3·6H2O
was completely dissolved, the precursor solution and the CeO2 were immersed, mixed vigorously with a stirrer for 20 min . Standing at room
temperature for 12 hours, then the composite was aged at 110 oC
for 12 h inside, taken out and ground, and finally calcined at 500 oC
for 5 h in a muffle furnace. The Ni loadings were 6.0 wt%, 8.0 wt%, 10.0
wt% and 12.0 wt% respectively.
Preparation of Ni-based catalysts with different supports
CeO2 and ZrO2 were used as carriers
to determine the water absorption rate, respectively. Two Ni(NO)3·6H2O
corresponding to 8.0 wt% Ni loading were weighed into different beakers
according to the water absorption rate of the support to Ni(NO)3·6H2O.
The deionized water is added to prepare the precursor solution, and the mass of
the two beakers is weighed. After the Ni(NO)3·6H2O is
completely dissolved, the precursor solution is impregnated with the two kinds
of carriers. A homogeneous mixture of the solution was obtained by stirring
with a magnetic stirrer for 20 min. Standing at room temperature for 12 h, then
the composite was aged at 110 oC for 12 h. And it was finally
calcined at 500 oC for 5 h by using a muffle furnace.
Evaluation
of catalytic activity
Test of the catalytic performance of the catalysts was
carried out in a fixed bed quartz reactor at atmospheric pressure in the
temperature range of 600 oC to 750 oC. The
catalyst (2g) was pretreated for 2 hours at 500 oC under
hydrogen stream. The reducing mixture gas was a composite of H2 and
Ar (H2: Ar=1:9, 300 mL/min) and brought in at a ramp rate of
10 oC/min from room temperature to 500 oC.
The schematic diagram of the fixed bed system is shown in
Fig. 1. The system consists of three parts: a feeding unit, a methane steam
reforming reactor and an analysis part. It is necessary to uniformly fill the
catalyst in the middle of the reactor before the reaction. First, a certain
amount of quartz sand is added to both ends of the flow channel. The particle size
of the quartz sand is close to that of the catalyst particles to avoid the
influence of internal diffusion, and then the catalyst sample is poured. The
quartz sand not only plays a supporting role, but also helps the gas in the
flow channel to be preheated. The outlet gases were detected online by a gas
analyzer (Gasboard 3100).
Samples
characterization
Powder X-ray diffraction data (XRD) were collected in the
Philips X’Pert PRO of PANalytical B.V. with Cu Kα radiation (λ= 0.1542 nm) and
a 2θ range of 10-90° to study the crystalline structure of the samples. The
morphologies of the synthesized catalysts were studied by scanning electron
microscopy (SEM, SUPRA 55 SAPPHIRE).
Samples
catalytic evaluation
Conversion of methane (XCH4) was calculated as
follows:
XCH4 ={[(nCH4)in-(nCH4)out]/(
nCH4)in}*100%
Hydrogen Selectivity (SH2) to the products was
determined as:
SH2(%) = (nH2)out/{(nH2)out + (nCO)out + (nCO2)out} * 100%
Hydrogen Yield (YH2) was determined as:
YH2 = SH2 * XCH4 * 100%
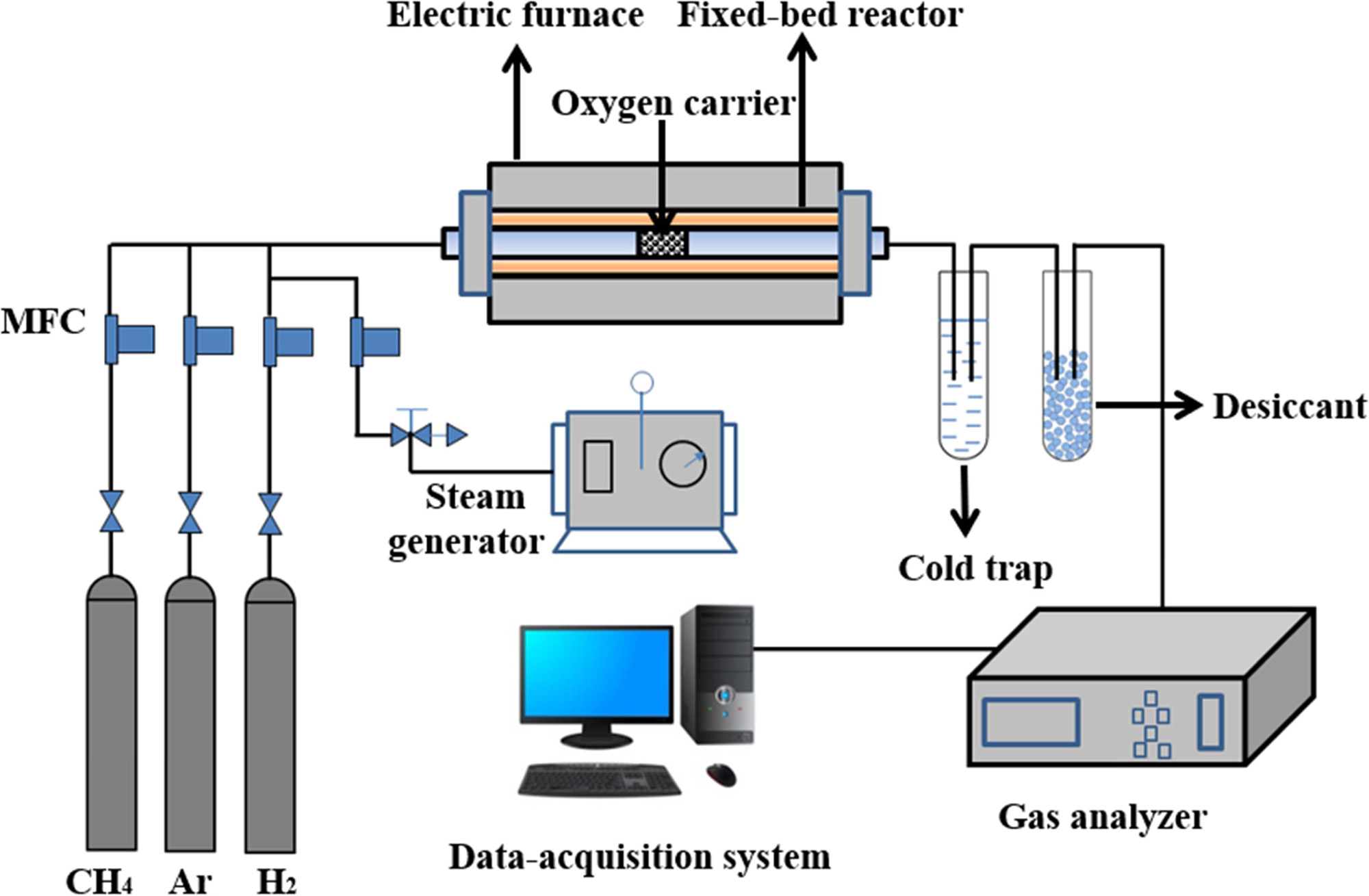
|
Fig. 1 Schematic diagram of the fixed bed system. |
Catalyst
characterizations
XRD diffraction result of the Ni/CeO2 with
different Ni loading of 6-12 wt% is displayed in Fig. 2. Compared
with standard cards in XRD database, the prepared Ni/CeO2
samples have a pure crystal structure. The diffraction peaks at 2θ = 37.4,
43.4 and 75.6° characterize the cubic NiO
phase (JCPDS file no.47-1049) [23, 24]. The
diffraction peaks corresponding to CeO2 at 2θ = 33.2, 56.6, 67.7,
and 83.7° are significantly increased.
Fig. 3 shows the morphology of the 10% Ni/CeO2
catalyst particles. It can be seen that the surface morphology of the carrier
CeO2 is porous, and NiO appears as a small particle with a size of
around 50 nm embedded in the CeO2 surface. The dispersion is
relatively high, and no agglomeration and sintering occur.
Catalytic
activity
Effect of different supports
Effect of different supports on catalytic activity of
Ni-based catalysts was studied in the fixed-bed system. The experimental
reaction temperature was 750 oC, the water-carbon ratio n (H2O):
n (CH4) was 3, and the space velocity was 1,500 h-1.
According to Fig. 4, it can be seen that under this condition, the Ni/CeO2
has a methane conversion rate and a hydrogen yield of 80.9% and 75.6%,
respectively. In addition, for the Ni/ZrO2 catalysts, the CH4
conversion rate and the H2 generation rate of the catalyst were
78.6% and 73.8%, respectively. Therefore, the nickel-based catalyst with CeO2
as a support has better activity regardless of the methane conversion rate or
the hydrogen yield. In addition, the hydrogen generation rate is also
correlated with the hydrogen selectivity. The results show that the hydrogen
selectivity of the catalyst with CeO2 as the support is 93.4%, and
the hydrogen selectivity of the catalyst supported by ZrO2 is 93.9%.
The selectivity will be slightly larger than the former, but the overall
difference is small.
Effect of different reaction temperatures
The methane steam reforming reaction is an endothermic reaction
and should be carried out in a high temperature atmosphere.
A suitable temperature is favorable for the reaction to generate hydrogen and
carbon monoxide. When the temperature is too high, it will cause carbon
deposition in the methane cracking, and the active component Ni will also be
sintered, which will affect the catalytic performance and the high temperature
will also threaten the life of the experimental equipment. If the temperature
is too low, carbon monoxide will be decomposed to form carbon deposits, which
is the main cause of carbon deposition in the catalyst, which in turn affects
the catalytic performance. Therefore, the reaction
temperature is a very important reaction condition in the MSR
reaction.
Fig. 5 demonstrates the comparison of performance of 10%
Ni/CeO2 catalysts at reaction temperatures of 650 oC,
700 oC and 750 oC (n(H2O):n(CH4)
=3, a space velocity of 1,500 h-1).
According to Fig. 5, it is found that the CH4
conversion rate and the H2 generation
rate increase with the increase of temperature. The methane conversion rates
are 69.1%, 74.6% and 82.4% at the temperatures of 650 oC,
700 oC and 750 oC, respectively. The CH4
conversion rate increased by 5.5% when the temperature was raised from
650 oC to 700 oC, and the CH4
conversion rate increased by 7.8% when the temperature was raised from
700 oC to 750 oC. The rate of increase is more
obvious when the temperature is raised from 700 oC to 750 oC.
As for the hydrogen generation rate, the corresponding hydrogen generation
rates are 45.5%, 59.1%, and 76.1% at the temperatures of 650 oC,
700 oC and 750 oC, respectively. Therefore,
750 oC was selected as the optimal temperature for Ni/CeO2
catalysts. The reaction temperatures of the following experiments were set to
750 oC.
Effect of loading different amount of active components
Generally, the larger the active component loading at the
same mass of the catalysts, the larger the particle diameter of
the active component and the corresponding catalytic
performance will be better, but when the loading is
too large, the active component will aggregate. The active
component in this experiment is Ni, which is easy to bring to aggregation and
sintering. If the load is too large, it is easy to produce carbon deposits, resulting
in a decrease in catalyst performance. Therefore, a suitable
Ni loading amount on catalyst plays a crucial role in MSR.
This part mainly investigates the effect of loading of
different active components on performance of Ni/CeO2 with Ni
content of 6%, 8%, 10% and 12% respectively (at temperature of 750 oC;
n(H2O): n(CH4) =3; and GHSV = 1,500
h-1).
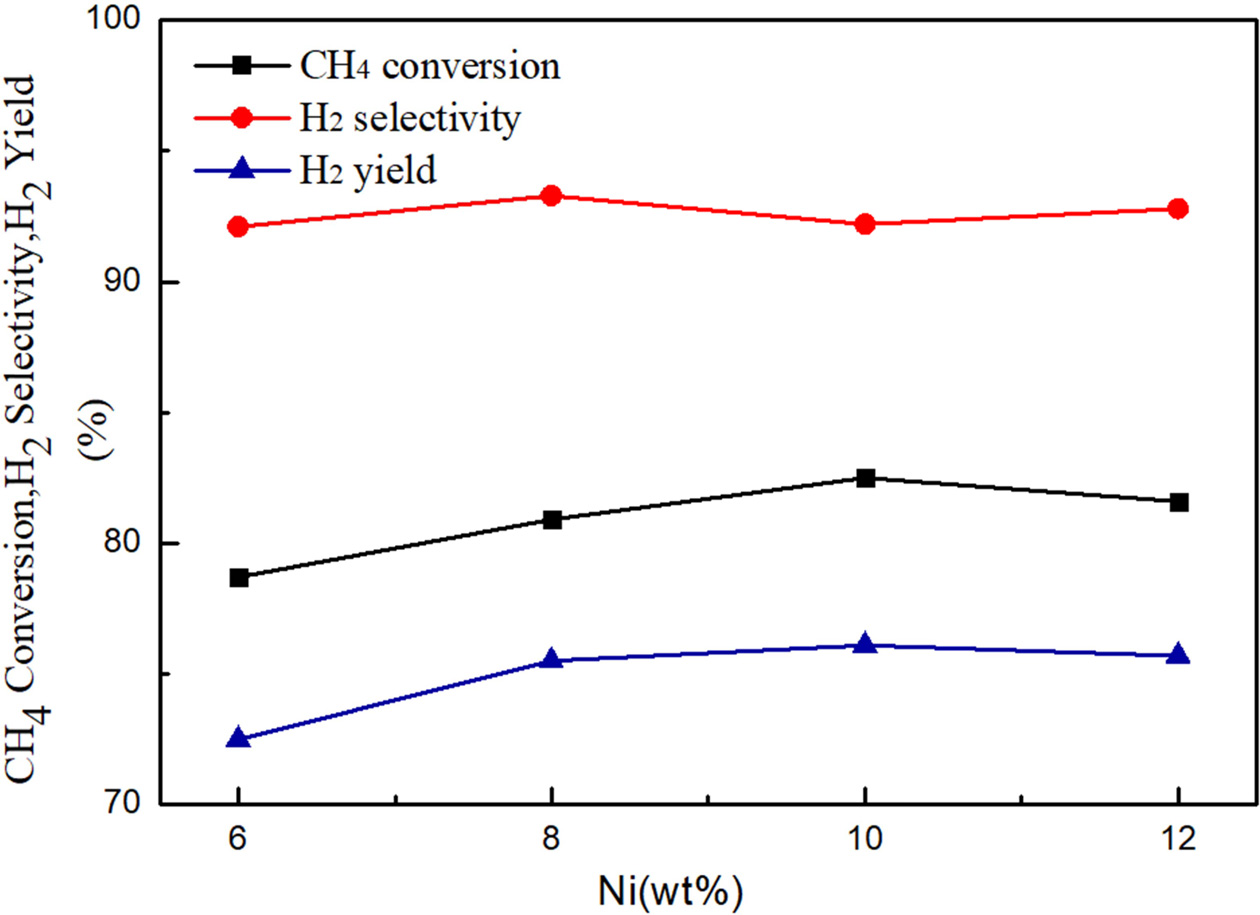
Fig. 5 shows the comparison of catalytic capability of 6% Ni/CeO2,
8% Ni/CeO2, 10% Ni/CeO2 and 12% Ni/CeO2 under
the same conditions.
As seen in Fig. 6, the CH4 conversion rate and
H2 yield are higher at the temperature of 750 oC.
The results indicate that the activity increases with the increase of Ni
loading from 6% to 10% and the reactivity decreases for the higher loading
content of 12% of Ni. The catalysts containing 10% Ni showed the
highest CH4 conversion rate and the H2 yield among
the samples. It can be seen that the CH4 conversion rate and the H2
generation rate have a significant increase in the Ni loading from 6% to 10%,
which is directly related to the increase of Ni loading, but when the Ni
loading is increased from 8% to 10%, the increase rate of hydrogen generation
rate in the range is less than 1%. It also can be seen that the hydrogen
selectivity decreases from 93.4% to 92.3% when the Ni loading is increased from
8% to 10%. When the Ni loading was continuously increased to 12%, it was found
that the CH4 conversion rate and the H2 yield were lower
than the Ni loading amount of 10%. The reason might be that the catalyst
agglomerates to produce agglomerates as the Ni loading becomes
larger. The carbon deposition reaction occurs to deteriorate the
catalytic performance.
Effect of GHSV
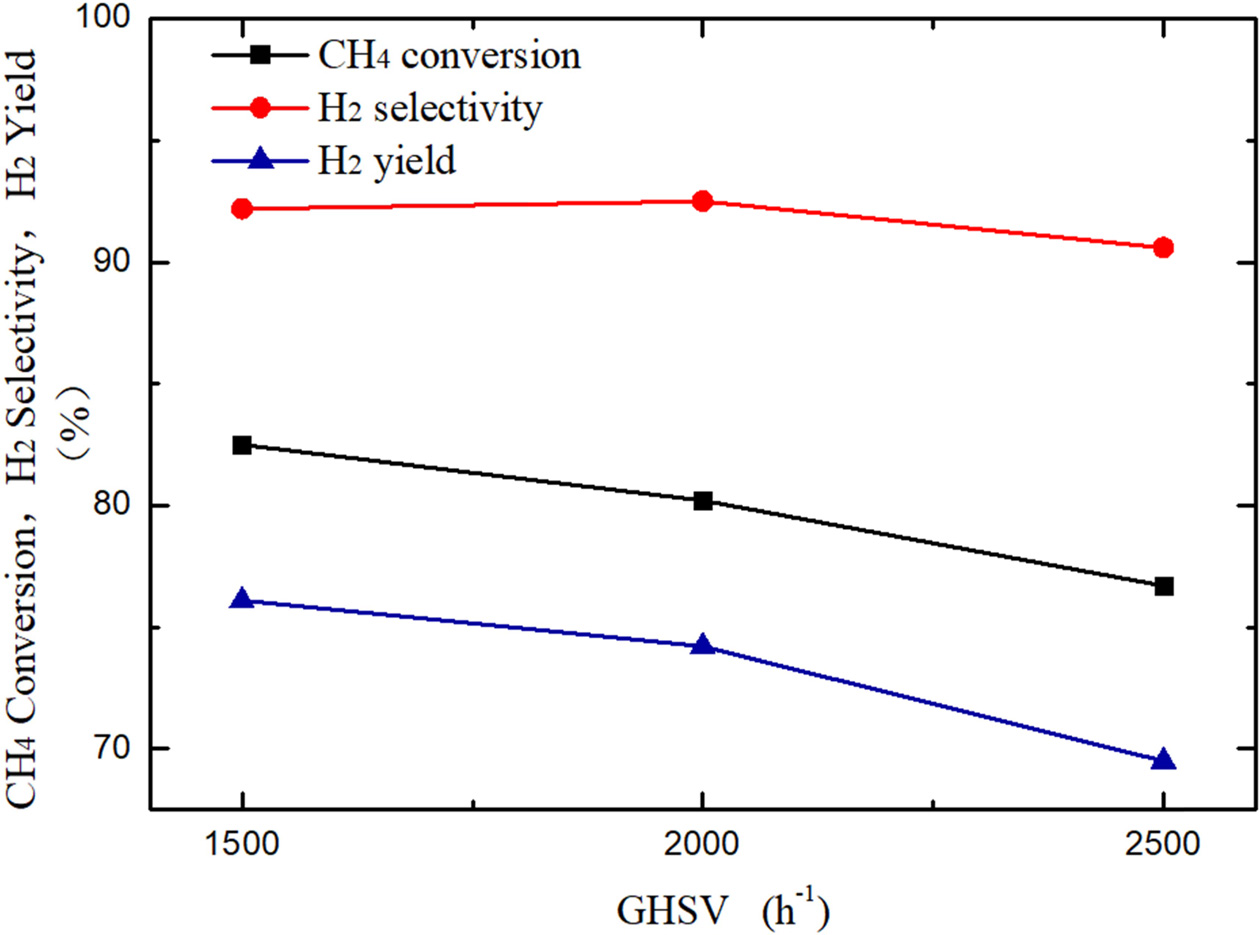
Fig.
7 shows the catalytic performance comparison of methane steam reforming reactions with different GHSV of 1,500 h-1,
2,000 h-1
and 2,500 h-1,
respectively. Results show that the CH4
conversion of methane steam reforming reactions at GHSV of 1,500
h-1,
2,000 h-1 and
2,500 h-1 were
82.5%, 80.2%, and 76.7%, respectively. In addition, the hydrogen
yield decreased by 1.9% with the GHSV changed from 1,500 h-1
to 2,000 h-1 ,
while the hydrogen yield decreased by 4.7% from 2,000 h-1
to 2,500 h-1,
which is the same as that of the methane conversion. Overall, GHSV of 1,500 h-1
corresponds to the best performance of the catalyst among the
conditions.
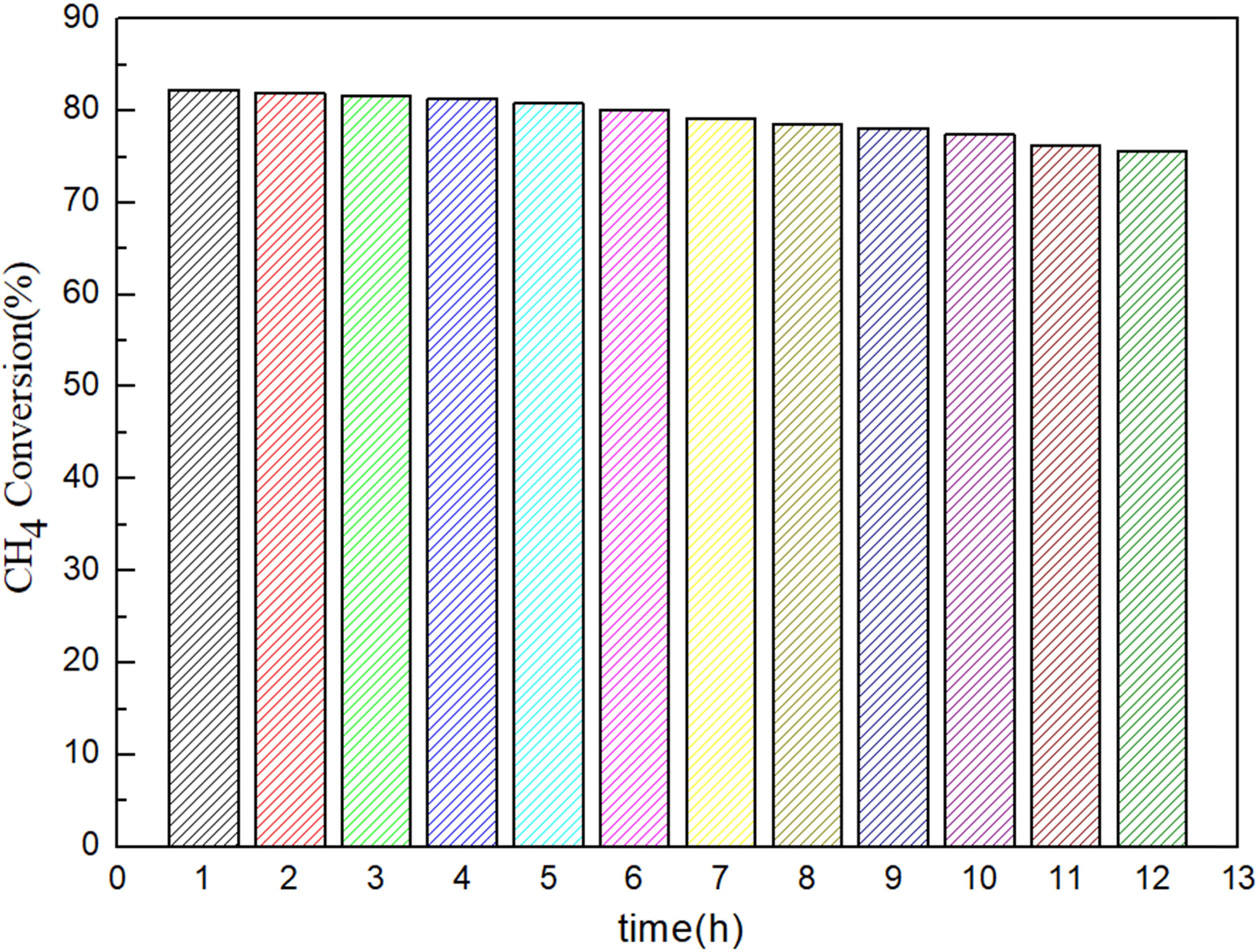
Stability tests
The methane conversion rate is an important parameter
for the stability of the catalyst. An experiment was conducted to evaluate the
stability of the catalyst at 750 oC for 12 h under flow
(10%Ni/CeO2, n(H2O): n(CH4) = 3, and GHSV =
1,500 h-1).
The experimental results are shown in Fig. 8. The catalytic activity remained
unchanged within 6 h, but the conversion of CH4 decreased slightly
(about 9%). This stability may be related to the small active NiO locations,
which are highly uniformly dispersed on the cerium support and have high
sintering resistance.
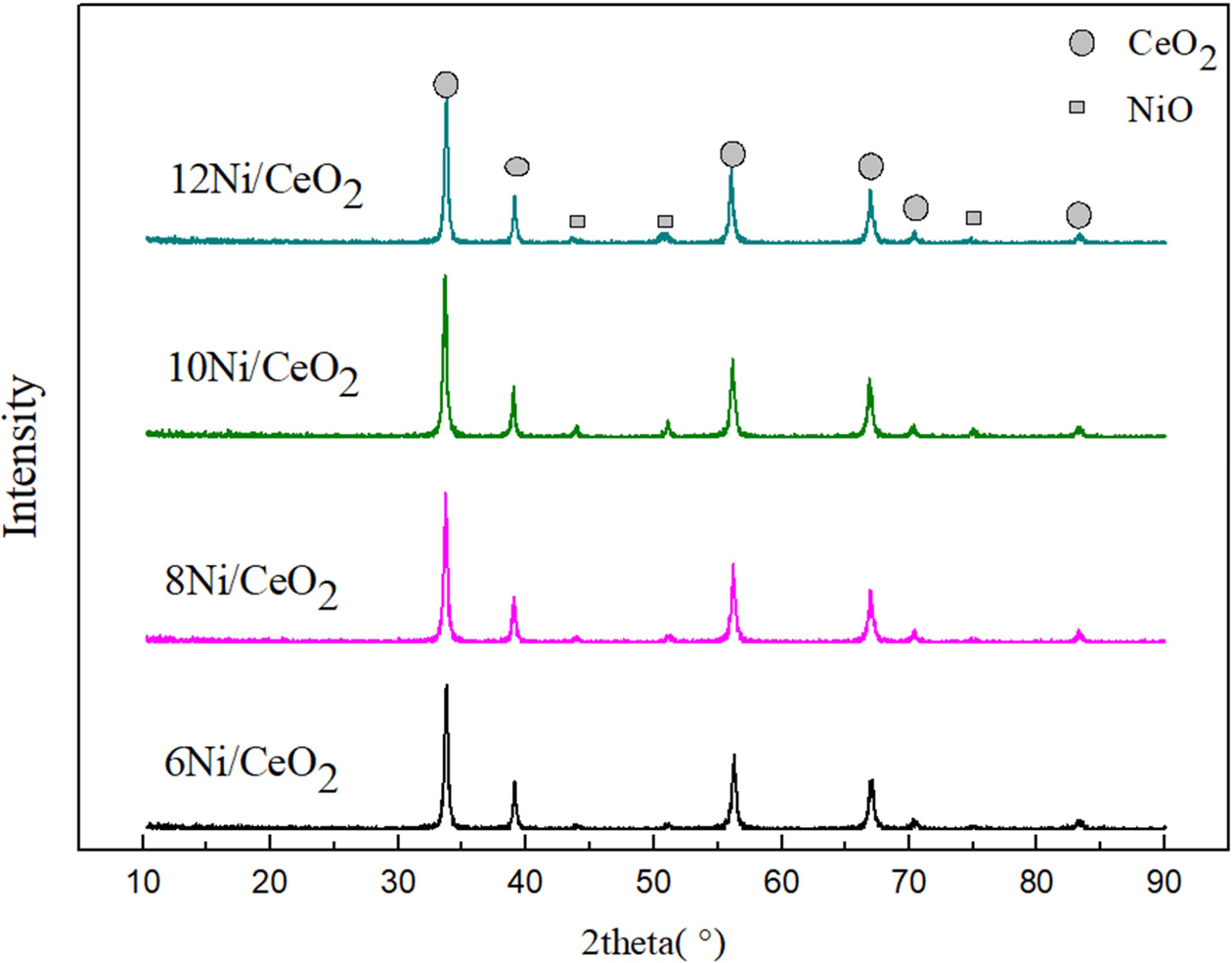
|
Fig. 2 XRD analysis of the Ni/CeO2 catalysts. |

|
Fig. 3 Results of Ni/CeO2 scanning electron microscopy with 10%Ni load. |
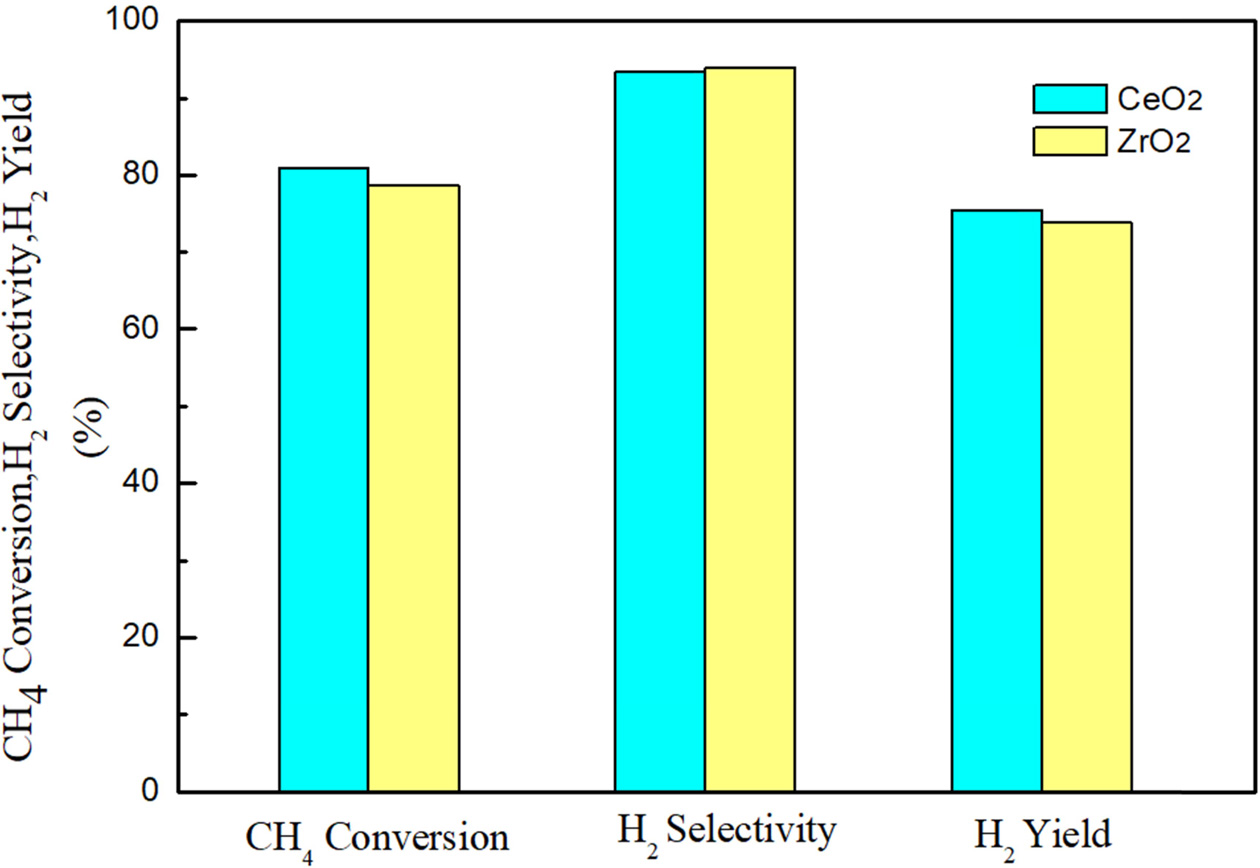
|
Fig. 4 Comparison of catalytic performance of and (n(H2O): n(CH4) = 3; GHSV = 1,500 h−1; T = 750 oC). |
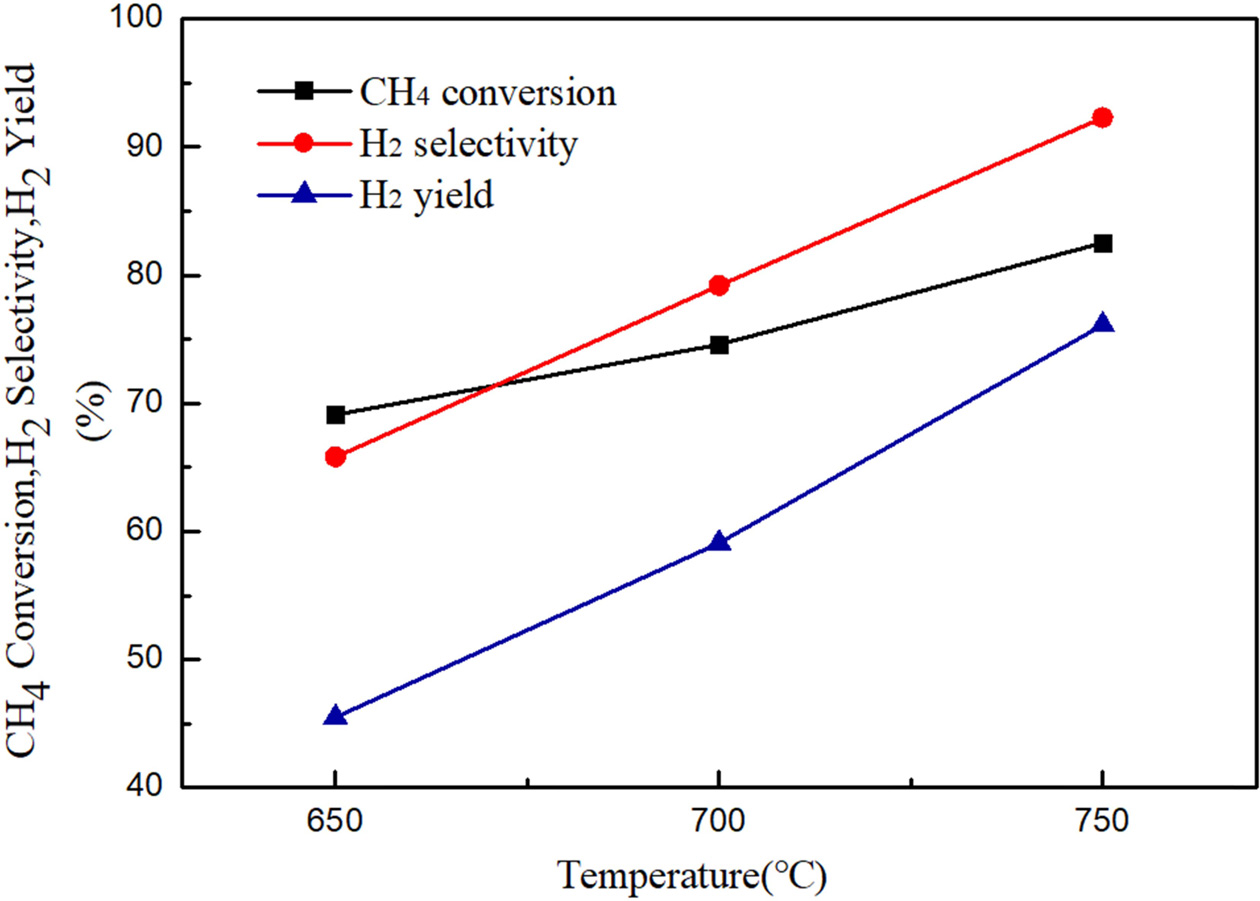
|
Fig. 5 Methane conversion and H2 yield at different temperature (n(H2O):n(CH4) = 3, GHSV = 1,500 h−1). |
|
Fig. 6 Methane conversion and H2 yield/selectivity for different wt % Ni loading (n(H2O): n(CH4) = 3; T = 750 oC). |
|
Fig. 7 Effect of GHSV on methane conversion and H2 yield/selectivity of 10Ni/CeO2(n(H2O): n(CH4) = 3; T = 750 oC). |
|
Fig. 8 Stability of catalysts for10%Ni/CeO2, Reaction conditions: T = 750 oC, n(H2O): n(CH4) = 3, GHSV = 1,500 h−1. |
Ni/CeO2 Nanocrystalline catalysts with
different Ni loadings (6, 8, 10, and 12%) were synthesized by a wetness
impregnation method for the methane steam reforming (MSR) process. The
catalytic performance of Ni/CeO2 catalyst was studied. XRD results
approved the formation of the nanocrystalline of the prepared catalysts.
It was shown that the 10 wt% Ni/CeO2 provided
the optimal catalytic activity among the catalysts with different Ni loadings.
The optimal reaction conditions for MSR by Ni/CeO2 catalyst was as
follows: 10 wt %Ni/CeO2, 750 oC, 1,500 h-1.
The stability test for 12 h at 750 oC demonstrated excellent
thermal stability of the resulted Ni/CeO2 catalysts.
This work was supported by the National Natural Science
Foundation of China Funding (No. 51606013), China Postdoctoral Science
Foundation Funding (No. 2019M651097 and No. 2019M651094), Fundamental Research
Funds for the Central Universities of China (No. 3132019187,
No. 3132019191 and No. 3132019327), and Natural Science Foundation of Liaoning
Province (No. 2019-BS-026 and No. 2019-ZD-0154).
- 1. W. Cai, L. Ye, L. Zhang, Y. Ren, B. Yue, X. Chen, and H. He, Materials 7 (2014) 2340-2355.
-

- 2. I. Dincer and C. Acar, Int. J. Hydrog. Energy 40 (2010) 11094-110111.
-

- 3. J.Matos, M. Rosales, R. Demir-Cakan, and M.M. Titirici, Appl. Catal. Gen. 386 (2010) 140-146.
-

- 4. T.L. LeValley, A.R. Richard, and Fan, M., Int. J. Hydrog. Energy 39 (2014)16983-17000.
-

- 5. O.A. Seyma, Int. J. Hydrog. Energy 35 (2010) 12821-12828.
-

- 6. A.C.W. Koh, L. Chen, W.K. Leong, B.F.G. Johnson, T. Khimyak, and J. Lin, Int. J. Hydrog. Energy 32 (2007) 725-730.
-

- 7. M. Rezaei, S.M. Alavi, S. Sahebdelfar, P. Bai, X. Liu, and Z.F. Yan, Appl Catal, B. 77 (2008) 346-354.
-

- 8. M.S. Fan, A.Z. Abdullah, and S.Bhatia, Int. J. Hydrog. Energy 36 (2011) 4875-4886.
-

- 9. A. Sardar, J.A. Mohammed, G. Ahmed, A.K. Abdelmoneim, and K. Mahmoud, Int. J. Hydrog. Energy 41[48] (2016) 22876-22885.
-

- 10. V.K. Jaiswar, S. Katheria, G. Deo, and D. Kunzru, Int. J. Hydrog. Energy 27 (2017) 18968-18976.
-

- 11. A. Berman, R.K. Karn, and M. Epstein, Appl. Catal. Gen. 282[1-2] (2005) 73-83.
-

- 12. R. Craciun, W. Daniell, and H. Knozinger, Appl. Catal. Gen. 230 (2002) 53-68.
-

- 13. V.P. De Souza, D. Costa, D. Dos Santos, A.G. Sato, and J.M.C. BuenoInt. J. Hydrog. Energy 37[13] (2012) 9985-9993.
-

- 14. N. Salhi, C. Petit, A.C. Roger, A. Kienemann, S. Libs, and M.M. Bettahar, Catal. Today 113[3-4] (2006) 187-193.
-

- 15. X. Fang, X. Zhang, Y. Guo, M. Chen, W. Liu, and X. Xu, Int. J. Hydrog. Energy, 41 (2016) 11141-11153.
-

- 16. R. Martınez, E. Romero, C. Guimon, and R. Bilbao, Appl. Catal. Gen. 274[1-2] (2004)139-149.
-

- 17. M. Cargnello, P. Fornasiero, and R.J. Gorte, Catal. Lett. 142 (2012) 1043-1048.
-

- 18. M. Ahmadi, H. Mistry, and B.R. Cuenya, J. Phys. Chem. Lett. 7 (2016) 3519-3533.
-

- 19. T. Montini, M. Melchionna, M. Monai, and P. Fornasiero, Chem. Rev. 116 (2016) 5987-6041.
-

- 20. W.S. Dong, H.S. Roh, K.W. Jun, S.E. Park, and Y.S. Oh, Appl. Catal. A Gen. 226 (2002) 63-72.
-

- 21. H.S Roh, K.W. Jun, and S.E. Park, Appl. Catal. A Gen. 251 (2003) 275-283.
-

- 22. T.J. Huang and M.C. Huang, Chem. Eng. J. 145 (2008) 149-153.
-

- 23. G.R. Gavalas, C. Phichitkul, and G.E. Voecks, J. Catal. 88[1] (1984) 54-64.
-

- 24. A. Zhao, W. Ying, H. Zhang, H. Ma, and D. Fang, Int J. Chem. Mol. Mat. Metal Eng. 5[11] (2011) 1039-1043.
-

 This Article
This Article
-
2020; 21(2): 263-268
Published on Apr 30, 2020
- 10.36410/jcpr.2020.21.2.263
- Received on Feb 10, 2020
- Revised on Mar 5, 2020
- Accepted on Mar 9, 2020
 Services
Services
- Abstract
introduction
experimental
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Qiuwan Shen
-
Marine Engineering College, Dalian Maritime University, Dalian, China
Tel: +86-18624118015 Fax: +0411-84728659 - E-mail: shenqiuwan@dlmu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.