- Ruthenium(II) dicarboxylated bipyridyl based metal organic polymer as a sensitizer for nanostructured TiO2 based dye-sensitized solar cells (DSSC)
Sathishkumar Chinnasamy and Sivasubramanian Ramanathan*
Electrochemical Energy Materials and Sensors Laboratory, NRIIC, PSG Institute of Advanced Studies, Coimbatore-4, Tamilnadu, India
In this work,
ruthenium(II)bis(2,2'-bipyridyl4,4'-dicarboxylicacid)(pyrazine)bis(tetrafluroborate)
[Ru(II)(dcbpy)2(pyz)]n(BF4)2n]
based metal organic polymer (RuMOP-Pyz-1), was synthesized at room
temperature under inert atmosphere and their performance in dye-sensitized
solar cells (DSSC) were studied. The metal organic polymer was prepared by
coupling pyrazine as linker units with the Ru(II) dicarboxylated based mono
metallic complex. The UV-visible absorption profiles covered a broad range of
absorption and the formation of polymer leads to shift in the absorption
wavelength. The metal mediated π-conjugation units in the polymer complex
exhibit a strong emission at λem = 552 nm with an excitation
wavelength of 395 nm. The synthesized metallo-polymer was employed as a
sensitizer in DSSC and the maximum power conversion efficiency (PCE) value of ɳ
= 1.507 % with short circuit current (Jsc) of 3.22 mA/cm2;
open circuit voltage (Voc) 0.684 V and Fill Factor
(FF) of 68.45 % under Air Mass (AM) 1.5 G simulated sunlight at a light
intensity of 100 mW/cm2 was obtained. The efficiency of the device
and its photovoltaic performances were found to be satisfactory. The enhanced
performance of the device is attributed to the presence of extended conjugation
of the metallo polymer which helps in a facile electron transfer from the HOMO
to LUMO of TiO2.
Keywords: DSSC, metallo-polymer, poly-Ru(II)dicarboxylated bipyridyl, pyrazine, photovoltaic, sensitizer
Dye sensitized solar cells (DSSC) are currently attracting significant
attention as alternative to conventional silicon solar
cells [1, 2] due to their low production cost, simplicity
of fabrication, lightweight, array of colour and high power conversion
efficiency (PCE). DSSC have been intensely pursued in last two decades
and has found many applications such as flexible electronics, transparent
conducting windows, etc. [3-5]. Typically DSSC is a multilayer device, where
nanocrystalline titania coated on a conductive substrate act as a
photoanode, platinized counter
electrode act as a photocathode. The photoanode (TiO2) with
the dye and the photocathode electrode were bridged
by a redox electrolyte, which mediates the charge transfer
between the electrodes. The sensitization is usually a metal based organic dye
which helps to improve the overall efficiency of DSSC [6, 7]. As
being a multilayer device, the most important components of
DSSC is the photosensitizer [8-10] and the efficiency of DSSC mainly depends on
the performance of the sensitizers [11].
In view of the potential application of DSSC, more number
of research works focused on each layer of DSSC like the photoanode, the photo
cathode, the dyes and the electrolyte. In the case of photo anode, various nanostructured ceramics
[12], semiconductor metal oxides with optimized
layer deposition and sintering process [13] were examined. Further,
in the case of photo cathode DSSC performances were enhanced by the addition of
conducting ceramic nanoparticles [14] and microporous ceramics [15] in
electrolyte systems. Specially, to
increase the near-infrared solar cell response by introducing transparent ceramics [16] and to get excellent
pigmenting materials through new ceramic dyes [17] has also been developed.
Predominantly, the ceramics based material for DSSC
research were played a vital role in various approach. Particularly, by
introducing TiO2 nanowires, carbon nanofibers
and porous carbon nanofibers with Pt catalysts were
reported effective results [18-22]. Hence, ruthenium
nanocrystals, composites based on TiO2 nanoparticle/nanowires,
size-selected titanium dioxide nanowires, mixed transition metal oxides TiO2
based photoanodes were also incorporated for efficient dye sensitized solar
cells [23-27]. By introducing various synthetic designs in dye structure and
blended structures of polymers [28] would increase the efficiencies of the
solar cells. So, enhancing the DSSC performance by introducing new functional
dyes is the focus of the current research [29]. Different types of dyes have
been used in DSSC, including natural [30], organic [31, 32] and
organometallic dyes [33] based on various transition elements
[34]. Predominantly, ruthenium complexes have received intensive interest due
to their favorable photo-electro-
chemical properties and high stability. Among these sensitizers
ruthenium polypyridyl based complexes have shown the best photovoltaic
performance due their better light harvesting properties upon absorption [35].
In particular, the dicarboxylated bipyridine based mononuclear
dyes N3 and N719 dye were considered as standard dyes due to their best
photovoltaic performance [36, 37]. So far several DSSCs
based on ruthenium complexes have achieved photovoltaic efficiencies of over
10% under standard measurement conditions and the highest value is about 12%
[38, 39].
Recently, inorganic perovskite sensitized solid state DSSC
have reached an efficiency of 20.1% [40]. On the other hand, the environmental
and health hazards owing to the occurrence of toxic components hold back their
commercial applications [41-43]. So, there is a high demand in the ruthenium
based sensitizers which achieves high efficiency with proper electronic and structural
properties [44]. In the case of standard sensitizers like N3
dye, the main drawback is the ambidentate thiocyanate ligand which will
coordinate either at the nitrogen atom or at the sulfur atom. So as to solve
the problems associated to NCS ligand based dyes, cyclo- metallated ruthenium complexes were
projected in the last three decades [45]. An immense effort has been made to
optimize the molecular structure of ruthenium complex by
varying ancillary ligands, typically bipyridines which can be
tuned by different substituent’s to modify their photochemical and
photophysical properties [46]. It has been well-accepted that
raising the molar extinction coefficient of a sensitizer is a
well-designed approach to enhance the photovoltaic performance [47].
Another important factor that hinders the performance
of DSSC is the poor thermal stability [48]. To overcome
this problem, supramolecular metallo-polymers with extended π-conjugation units
were employed. These metallo-polymers exhibit the combination of both the
properties of organic and organometallic compounds [49]. Moreover, these dyes
show not only competent light harvesting efficiency but also
an extended absorption with high molar absorption
coefficient. Besides an effective intramolecular spatial charge separation of
the excited state are critically important for better
performance. Ruthenium based metallo-polymers which consists more
number of conjugated units has various advantages, such
as long-lived metal to ligand charge transfer (MLCT) and
ligand centered redox process [50]. Employing the organic polymeric materials
as sensitizers in DSSC has been recently recognized and comparatively modest research
work done has been carried out [51]. In this work, we
report the synthesis and characterization of ruthenium (II)bis(2,2'-bipyridyl4,4'-dicarboxylicacid)
(pyrazine) bis(tetrafluroborate) based metal
organic polymer (RuMOP-Pyz-1) and their efficacy as a photosensitizer is
examined.
Materials
and methods
RuCl3.3H2O, 4,4'-dimethyl
2,2'-bipyridine (bpy) (<99%), 10% palladium on charcoal, Pyrazine
(<99.5%), silver tetrafluroborate (AgBF4) (<99%), P25
TiO2 powder (<99%), TiCl4 solution, chloro platinic
acid (H2PtCl6), ethanol, N-methyl pyrrolidine (NMP),
acetyl acetone and diethyl ether were purchased from Sigma Aldrich and were
used as received. Triton X - 100 (<98%) were purchased from Merck and used
as received. All the synthetic reactions were performed
under inert condition. The solvents employed for the
synthesis were reagent grade which were freshly distilled and degassed prior to
use.
Characterization
The absorption spectra were recorded in DMF by using
Shimadzu UV-1800 UV-VIS spectrophotometer (Japan) with
1 cm2 quartz cell. Emission properties were studied by
using Shimadzu RF-6000 spectrofluoro-
photometer (Japan). FTIR spectra were assessed in the
400 ~ 4000 cm-1
region by using IR Affinity FTIR, (Shimadzu, Japan). The AFM analysis was
obtained by using NT-MDT (Ntegra Aura, NTMDT Co, Russia). FESEM
analysis were taken by FESEM – (SIGMA HV –
Carl Zeiss, Bruker Quantax 200 – Z10 EDS Detector). Raman
analysis was performed by a micro Raman spectrometer (Horiba Jobin-LabRam-HR
UV-vis μ-Raman spectrometer, Finland) at ambient temperature with Argon laser
with an excitation wavelength of 514 nm equipped with CCD detector.
Synthesis
The metal organic polymer (RuMOP-Pyz-1) was
synthesized by a one-pot reaction. The metal organic polymer was obtained by
reacting the mono metallic complex with the linker unit as follows. 5 mL of dry
ethanol solution of Ru(dcbpy)2Cl2 (0.0807 mmol), 5 mL of
AgBF4 (0.1613 mmol) in ethanol was added under argon atmosphere and
stirred for 2 h at room temperature. The precipitated AgCl was filtered and to
the obtained solution, 5 mL of pyrazine (0.0884 mmol) in ethanol was added drop
wise and stirred for 1 h. The metallo-polymer was filtered, washed with ethanol
(50 mL). Further purification was obtained by treating it with N-methyl
pyrrolidine (NMP) and precipitating it with addition of diethyl ether. Finally,
the precipitated metallo-polymer was dried under vacuum at 40 oC
for 8 h. The metal organic polymer (RuMOP-Pyz-1) was obtained as
a dark red-brown solid (yield: 97%). The detailed synthetic pathway was
illustrated in Scheme 1.
Analysis of [Ru(II)(dcbpy)2(pyrazine)]n(BF4)2n]
(RuMOP-Pyz-1): UV-Vis
(3.5 × 10-5 M
in Ethanol): λmax/nm 535. Emission (λmax)
553 nm excited at 395 nm. Melting Point: >450 ºC. FTIR (KBr disk) ν/cm-1:
3452 (b), 2108 (m), 1984 (m), 1751 (s), 1630 (s), 1604 (s), 1513 (s), 1491 (s),
759 (s).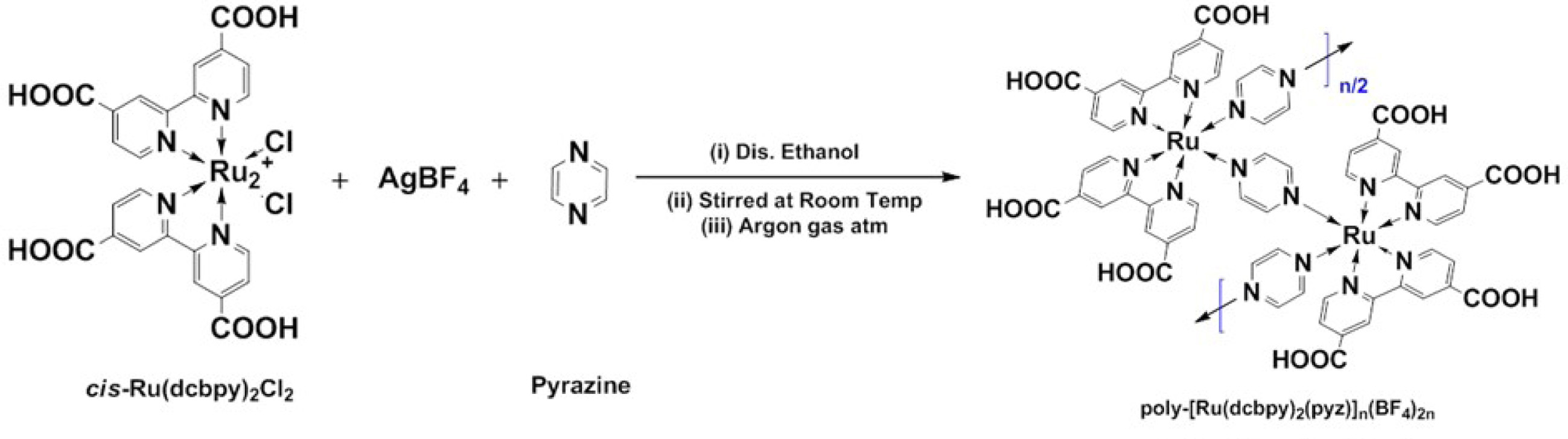
Scheme 1. Depicts the synthesis of poly-[Ru(dcbpy)2(pyz)]n(BF4)2n, coded as (RuMOP-Pyz-1), where (dcbpy) - 2,2'-bipyridyl 4,4'-dicarboxylic acid, (pyz) - pyrazine (linker unit), cis-Ru(II)(dcbpy)2Cl2 - cis-dichlorobis(2,2'-bipyridyl 4,4'-dicarboxylicacid)ruthenium(II) and poly-Ru(dcbpy)2(pyrazine)]n(BF4)2n - poly [Ruthenium(II) bis(2,2'-bipyridyl4,4'-dicarboxylicacid) (pyrazine)bis tetrafluroborate)].
Fabrication
of DSSC
Fluorine doped tin oxide glass (FTO) (~7 Ω/sq,
purchased from Sigma Aldrich) plates were cleaned by ultrasonication with
water, isopropanol and finally dried under nitrogen atmosphere. The cleaned
substrate was heated up to 500 ºC for 25 min to remove organic contaminants and
cooled down to room temperature. The compact layer of titania
nanoparticle (pre treatment) was coated by immersing the FTO
plates in a 40 mM aqueous TiCl4 solution at 70 ºC for 30 min
and followed by a series of sintering steps ((i) 325 ºC for 10 min, (ii)
375 ºC for 10 min, (iii) 450 ºC for 15 min, and (iv)
500 ºC for 30 min). Then by using mortar and pestle, P25
TiO2 powder, acetyl acetone, Water and Triton X - 100
ingredients were grinded to paste. Then the prepared paste was
coated on the pretreated (compact layer) FTO substrates by doctor-blade method
and 12 µm thick film (active layer) was achieved. The TiO2 pastes coated
FTO plates were subjected to the above mentioned similar
sintering steps. After cooling to room temperature, the
TiO2 electrodes were sensitized with metallo polymeric
dye (RuMOP-Pyz-1) by immersing the electrode in 20 mM
sensitizer solution in DMF. Similarly photocathode platinum
coated FTO were prepared by using 0.005 M of H2PtCl6
solution. By using spray gun, the prepared solution was spread on cleaned
pre-heated FTO plates at 50 ~ 60 °C. After the coating the slide
was kept on hot plate at 380 °C. In air atmosphere the pretreated plates
were kept in hot plate and the temperature of the hot plate was gradually increased
from 100 °C to 380 °C. The plates were pre-annealed at 380 °C for 30
min and then slowly cooled down to 80 °C in air
atmosphere. The coated plates can be used at any time, by giving the
photocatalytic regeneration activity by pre annealing it on hot
plate at 380 °C for 30 min.
Photovoltaic
characterization
The current-voltage (I-V) characteristics were recorded
by applying an external potential bias to the cell while recording the
generated photocurrent with a Keithley model 2420 digital source meter. The
light source was a 450W xenon lamp (Oriel) equipped with a Schott K113 Tempax
sunlight filter (Praezisions Glas & optic GmbH) in order to match the
emission spectrum of the lamp to the AM 1.5G standard. The metal organic
polymer (RuMOP-Pyz-1) sensitized DSSC was tested under simulated AM1.5G
illumination (power 100 mW cm-2)
using standard 1-Butyl 3-Methyl Imidazolium Iodide [BMII] electrolyte
containing the iodine/tri iodide couple as the redox shuttle.
Synthesis
of metal organic polymer dye (RuMOP-Pyz-1)
The synthesis of RuMOP-Pyz-1 was prepared by
the reaction of cis-dichlorobis(2,2'-bipyridine
4,4'-dicarboxylic acid) ruthenium (II), cis-Ru(dcbpy)2Cl2
with AgBF4 (the chloride is removed by the precipitation of AgCl)
and the metallo-polymer is formed by the reaction with pyrazine
which acts a linker. It is found that the obtained metal
organic polymer is soluble in polar aprotic solvent like DMF, DMSO, etc..
UV-Visible
Spectroscopy
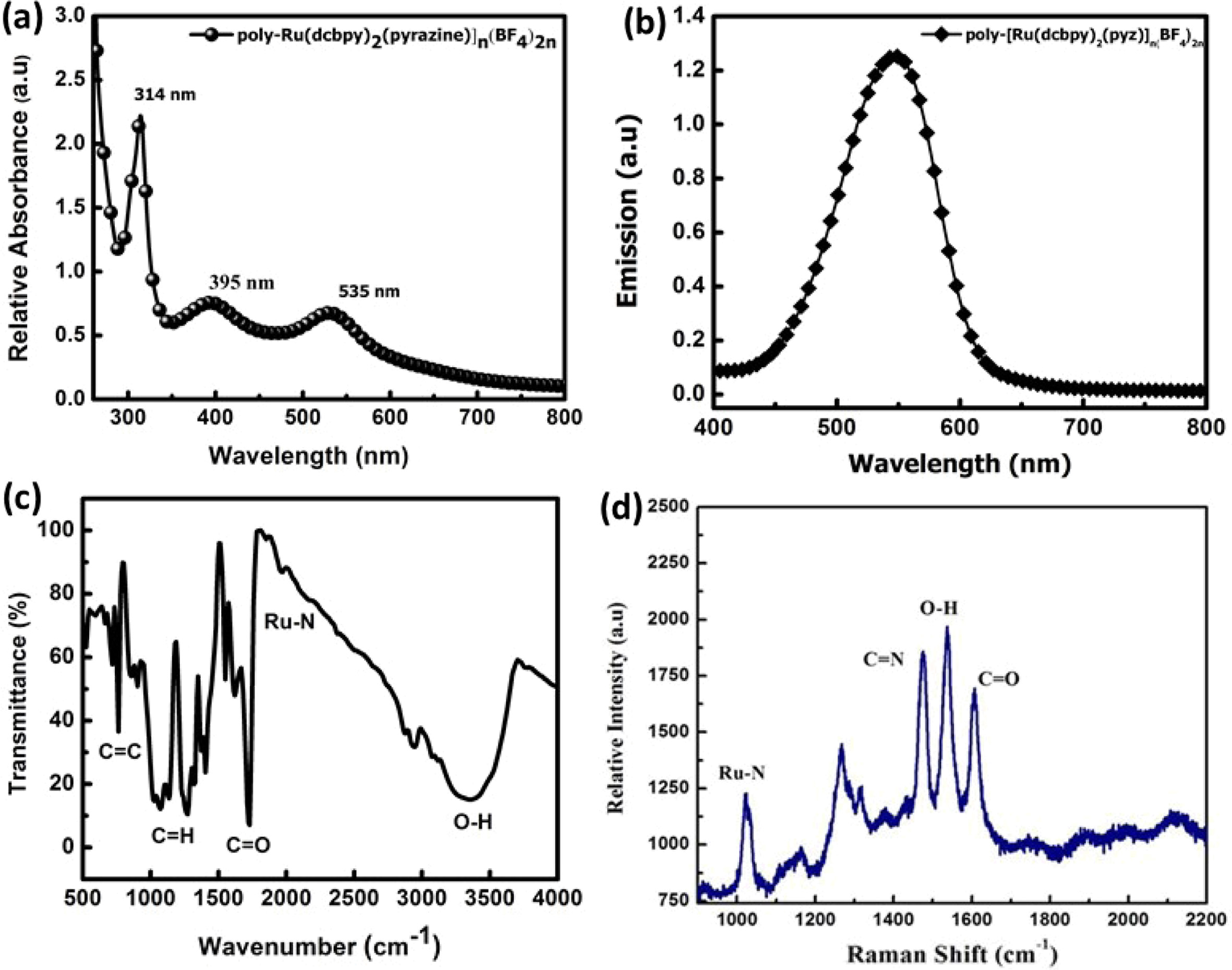
The UV-vis absorption spectrum of the metal organic polymer
(RuMOP-Pyz-1) in DMF solutions were shown in the Fig.
1(a). The absorption spectrum shows a sharp band at 314 nm, which corresponds
to the ligand π - π* transitions [52, 53]. A very
broad and more intense band was obtained in the visible and near
visible regions at 535 nm and 395 nm for the compound RuMOP-Pyz-1
respectively. The bands in the visible and near visible region bands
were assigned to metal-to-ligand charge transfer (MLCT) band of (d - π*) transition
and π - π* transition of the conjugated linking units
respectively. It is worth noting that compared with the other Ru(II)
dicarboxylated based complexes, the synthesized metal organic polymer
showed extended absorption beyond MLCT. The reason for this red
shift could be due to the presence of extended conjugated systems with the
electron withdrawing carboxylic acid groups, which lowers the energy of the π*
orbital of the ligand [54].
Emission
properties
The excitation of RuMOP-Pyz-1 was carried
out at 395 nm and the corresponding emission spectrum was obtained at 553 nm as
shown in Fig. 1(b). This broad emission spectrum is due to the lower π* orbital
of the 2,2'-bipyridyl-4,4'-dicarboxylic acid ligand and it is mainly due to the
presence of electron-withdrawing substituent which is expected to give
the longer lifetime. It has been noted that as the metal extended
π-conjugation increases, a strong and broad
phosphorescence spectrum was obtained [55].
FTIR
and raman spectroscopy
The FTIR spectrum of RuMOP-Pyz-1 in Fig.
1(c) showed a strong band in the region of 3,400 cm-1,
due to the presence of O-H group of the carboxylic acid moiety. The relatively
strong absorption at 1,711 cm-1
corresponds to the stretching vibration mode of C=O bond and the bands at 1,362
cm-1 and
1,603 cm-1
are due to the symmetric and asymmetric stretching in the C=O and C-H bands
respectively. Due to the N-coordination from the pyrazine unit a
stretching vibrational modes at frequency of 2,011 cm-1
for the γ(Ru-N) band was obtained [56]. Furthermore, the C-C bond
linkage in the aromatic skeleton was confirmed by the absorption peak
at 804 cm-1.
The Raman spectrum in Fig. 1(d) was obtained for
the ruthenium (II) dicarboxylated bipyridyl based metal
organic polymeric dye RuMOP-Pyz-1, at an excitation wavelength of 430
nm. The vibration modes at 1,102 cm-1,
1,471 cm-1,
1,539 cm-1, 1,610
cm-1
corresponds to Ru-N, C=N, O-H, C=O groups of the
synthesized metal organic polymer respectively.
DSSC
Fabrication – Electrode preparation
The typical DSSC and the detailed preparation involved in
each layer of DSSC is as follows, as is given in the Scheme 2. The photo anode
being the nanostructured TiO2 sensitized with the metal organic
polymer dye and photocathode being the Pt coated FTO and the cell was
fabricated with BMII electrolyte as redox couple. With light irradiation, the
sensitizer absorbs the dye and moves to excited state where by shuttling
the electron to TiO2 which in turn pass through
the circuit to photocathode. Further the electrolyte helps in shuttling the
electron from photocathode to dye. The overall efficiency of the device is
deduced using the following equation,
Efficiency (ɳ) = (Voc * Jsc)/(P) * FF (1)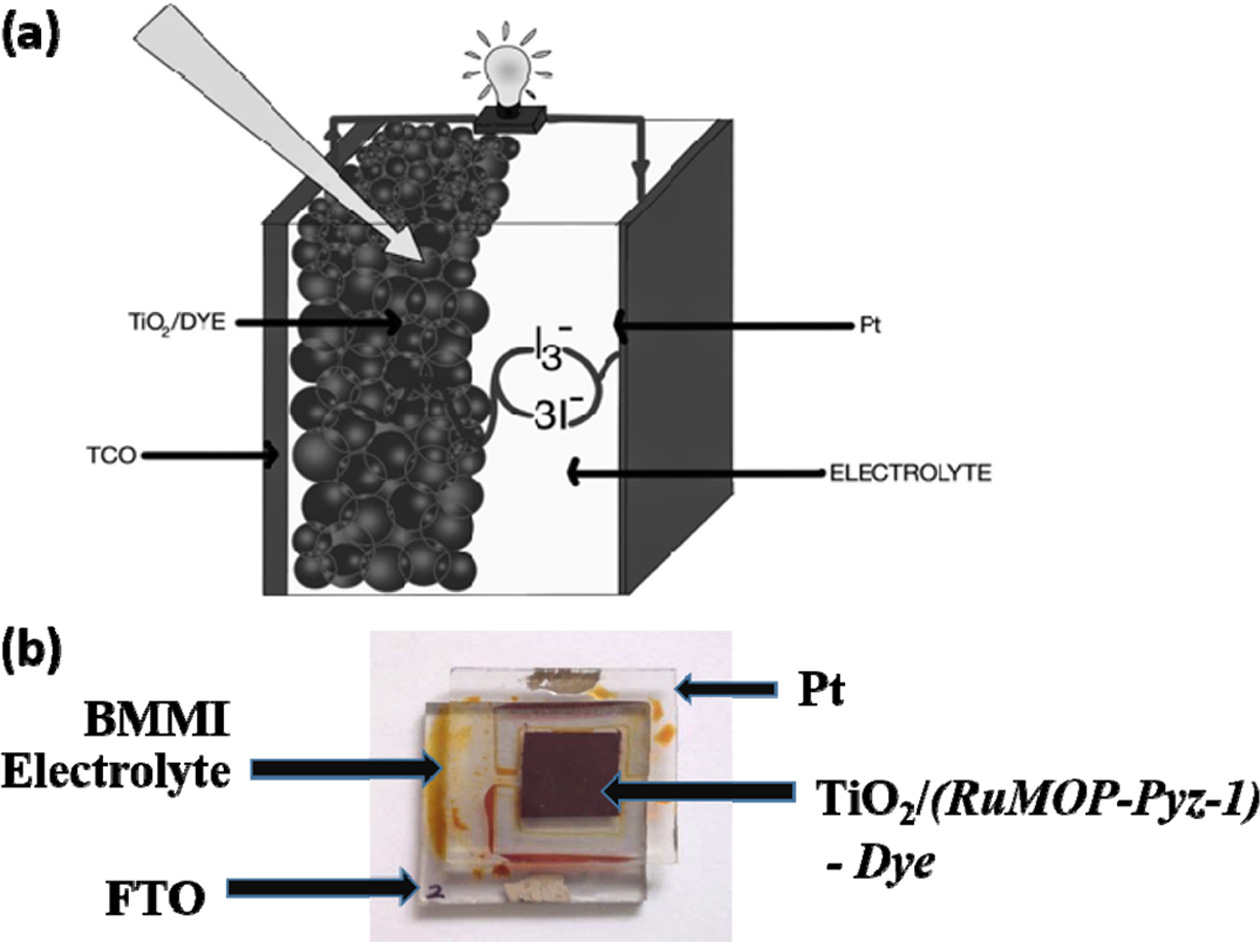
Scheme 2. (a) Schematic representation of typical DSSC device; (b) Real time DSSC device in which the sensitizer is (RuMOPPyz-1) (poly-ruthenium(II)bis(2,2'-bipyridyl4,4'-dicarboxylicacid) (pyrazine)bis(tetrafluroborate) - poly-[Ru(dcbpy)2(pyrazine)]n(BF4)2n).
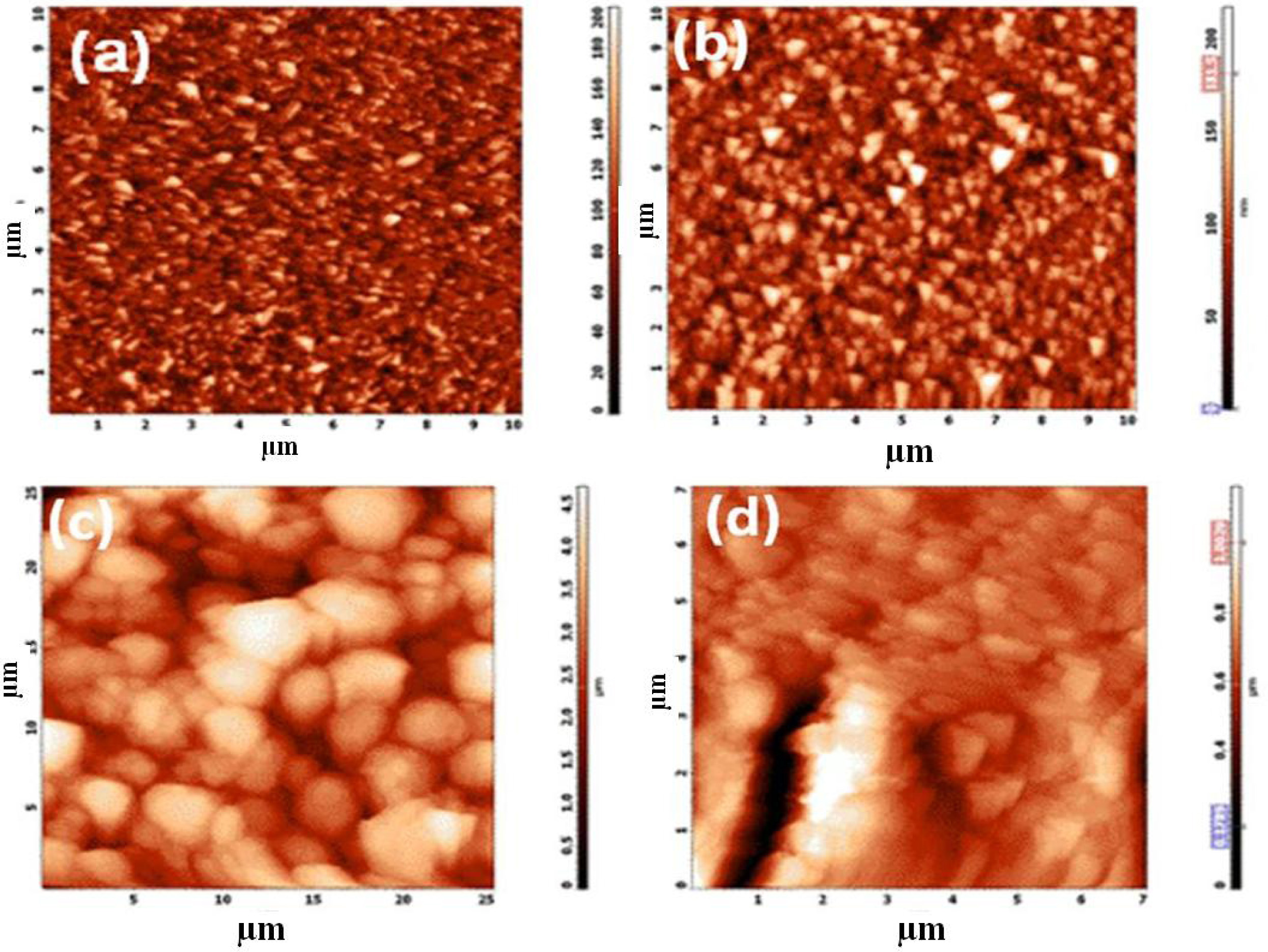
AFM and FESEM analysis
The AFM 2D images of the nanostructured coating of
TiO2 nanoparticles on FTO glass plate and its adsorption
with the metal organic polymer dye RuMOP-Pyz-1 were clearly seen from
the Fig. 2. The surface topo-
graphy of the FTO, compact layer, active layer and dye adsorption were
shown in the Fig. 2(a, b, c & d) respectively. To determine the average
pore size, ImageJ analysis software, were utilized and the average diameter of
the pores in between the TiO2 nanoparticles to be ~10 and ~30 nm for
compact layer and active layer coating of TiO2 nanoparticles . The
root-mean-square roughness (RMS) values of the each layer were 70, 90, 170 and
650 nm. The increased value of RMS indicates that reduced amount reflectivity
of incoming light confirms the formation of TiO2 nanoparticles on
FTO.
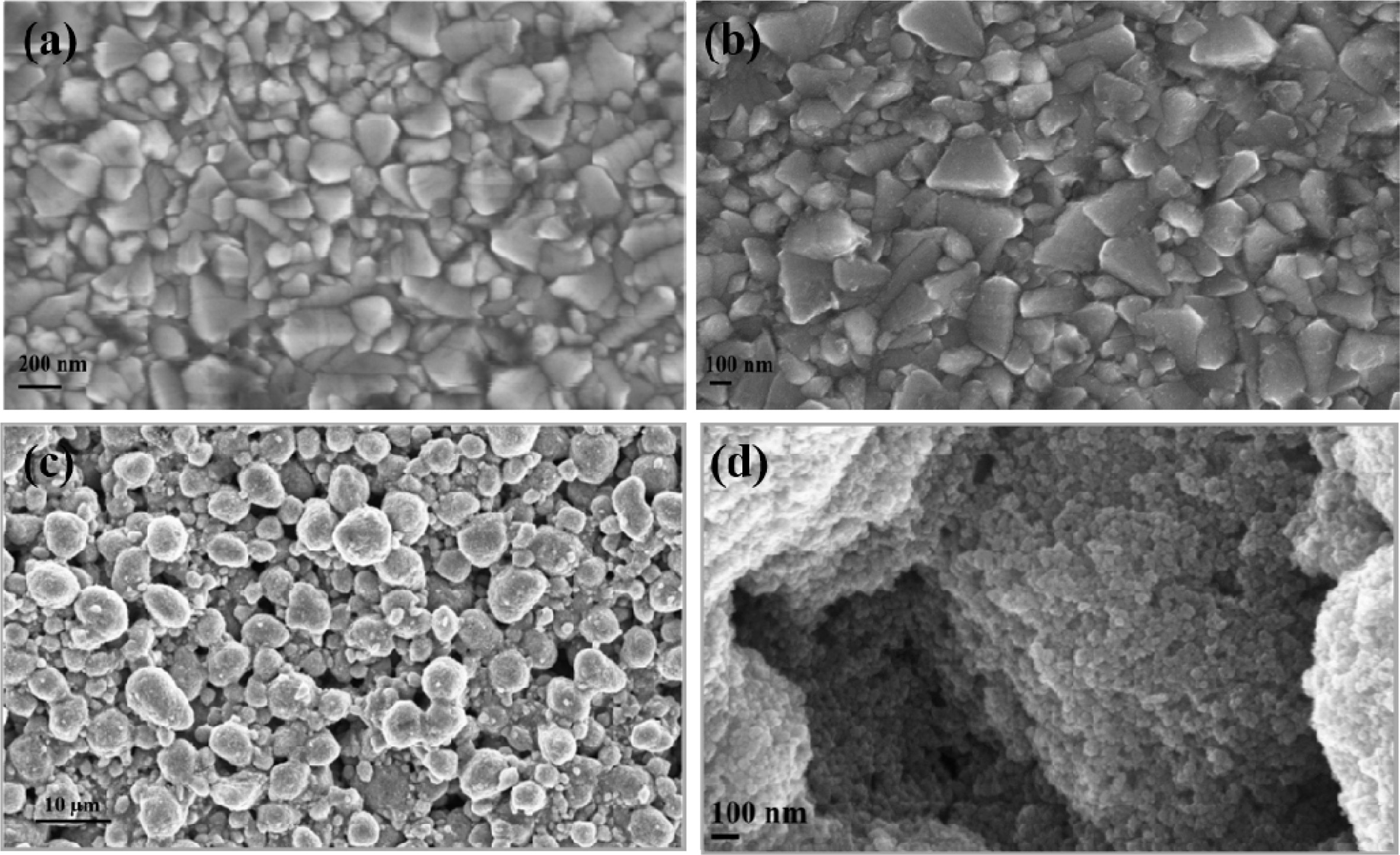
The FESEM image of the bare FTO, Compact layer and active
layer coating of nanocrystalline titania on FTO were clearly shown in Fig. 3.
The FTO deposition on glass substrate was shown in Fig. 3(a). The compact layer
of deposition of titania nanoparticle as pretreatment for
the photoanode as barrier layer on FTO was achieved (Fig.
3b) by dipping the FTO plates in a 40 mM aqueous TiCl4
solution and followed by a series of sintering steps. In Fig. 3(c) globular
structures with aggregated nanoparticles were noted on the above of compact
layer as TiO2 photoanode. In particular, Fig. 3(d)
depicts the availability of porosity for the deep penetration of
the dye within TiO2 was observed which enhances the easy electron
transfer from LUMO of the dye to the HOMO of the TiO2
nanoparticles. The available porosity in between the TiO2 nanoparticles
is very essential for the effective dye adsorption.
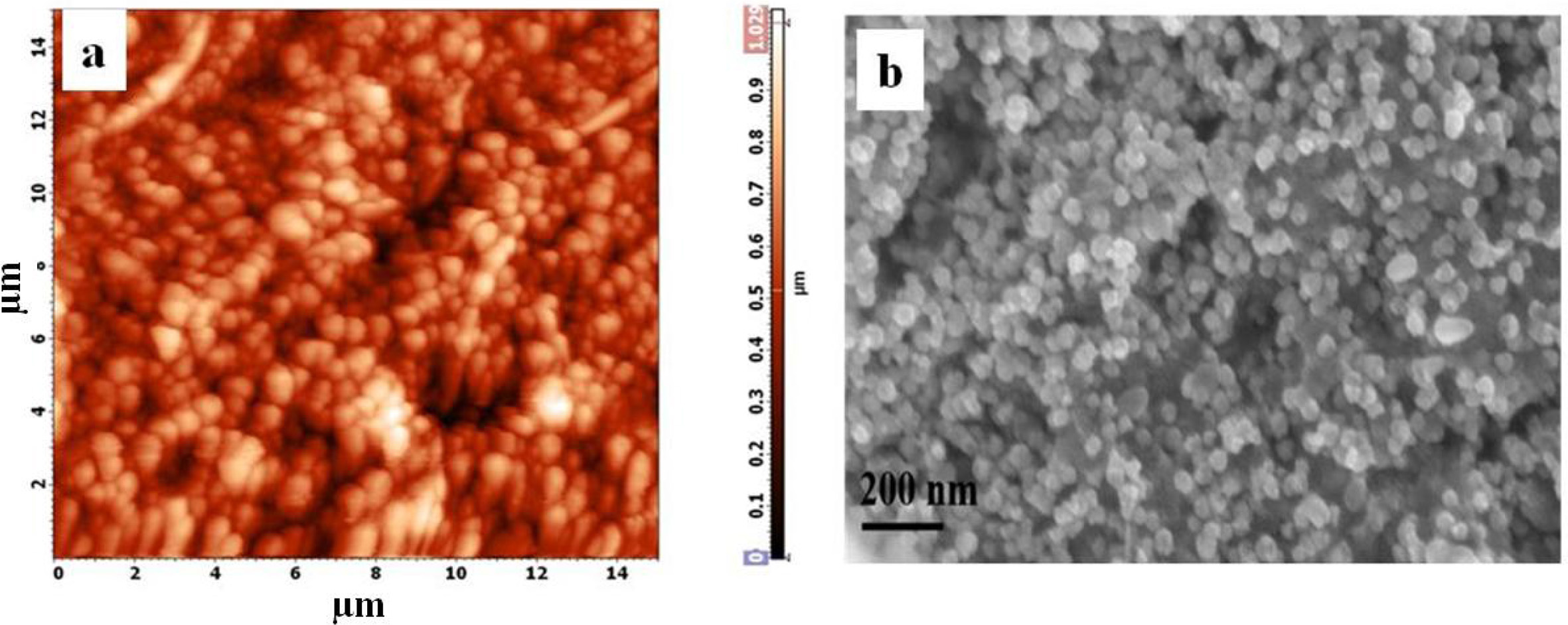
The photo cathode preparation and its corresponding AFM
and FE-SEM images were given in Fig. 4(a & b) respectively. The AFM 2D
image displays the uniform distribution of Pt nanoparticles on FTO. The high
resolution image of the FESEM (Fig. 4b), provides information of the platinum
electrode prepared by spray coating. The layer appears conformal with the conductive
oxide surface and show good layer uniformity. Hence, we
can see how the platinum nanoparticles are localized on the FTO surface.
Importantly, the coated particle was interconnected continuously and produces
highly porous structures of Pt nanoparicles.
I-V
characteristics
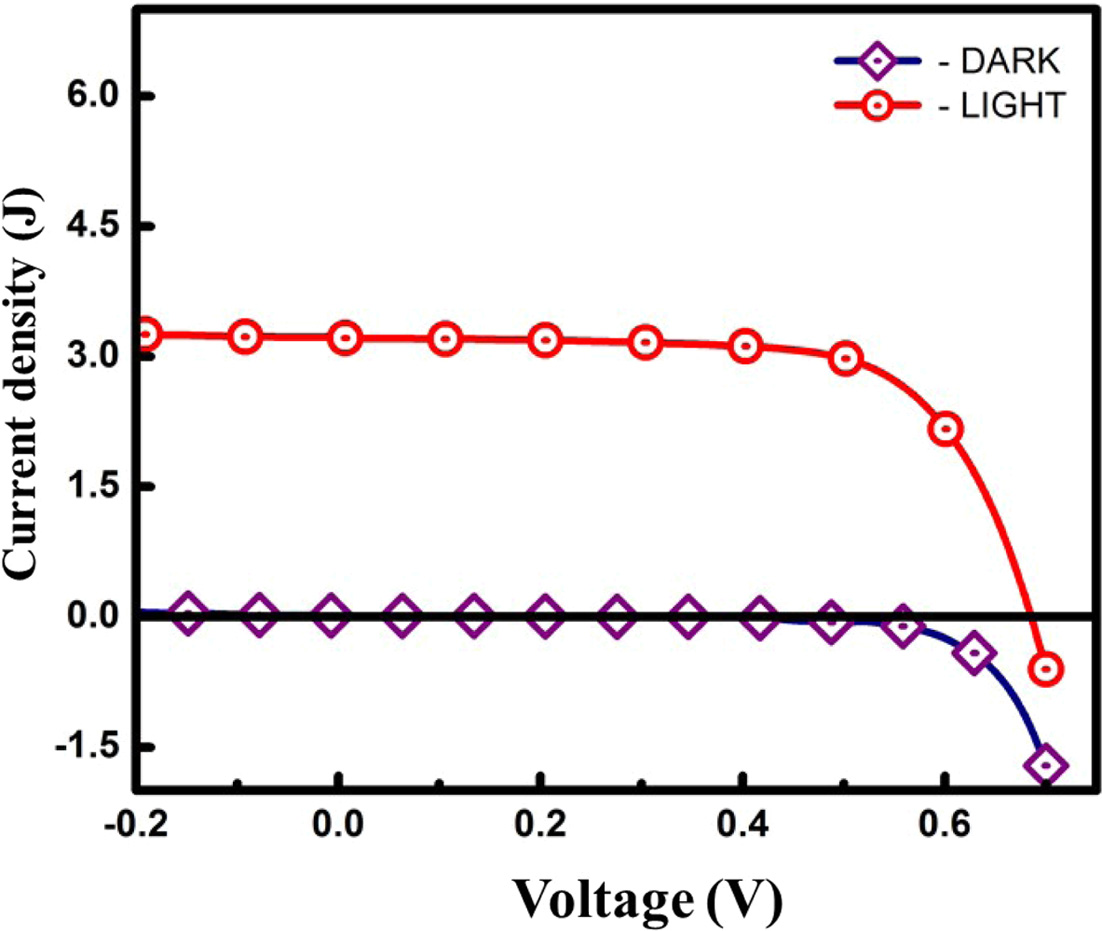
The current density (J) versus voltage (V)
curves of the DSSC is shown in Fig. 5. The photovoltaic measurements
of open circuit voltage (Voc), short circuit current density
(Jsc), fill factor (FF), and the PCE (ɳ) values
were shown in Table 1. The DSSC fabricated with the synthesized metal organic
polymer (RuMOP-Pyz-1), exhibited the photo
conversion efficiency value (ɳ) of 1.51% with short
circuit current (Jsc) 3.22 mA/cm2; open circuit
voltage (Voc) 0.684 V and Fill Factor (FF)
68.45%. When compared to the reported ruthenium dyes, 2,2'-bipyridine
4,4'-dicarboxylic acid based supramolecular ruthenium main-chain metal organic
polymer which has pyrazine conjugated bridging ligand
acting as linker unit, has better photovoltaic properties.
By using linear ligands through the conjugated linker
units, the charge separation distance and the charge
recombination effects in DSSCs can be controlled. Normally,
the carboxylic group in dcbpy moiety will be attached to the TiO2 surface
and thus by acting an electron withdrawing substituent in the bipyridine
ligand. So, in this synthesized metal organic polymer which has more number of
dicarboxylic acid group (dcbpy) throughout the polymer provides the necessary coupling
of the LUMO level of dye with the conduction band of
titanium dioxide, in order to promote efficient quantum injection.
|
Fig. 1 (a) UV-Vis spectra of (RuMOP-Pyz-1) in DMF; (b) Emission spectrum (RuMOP-Pyz-1) in DMF; (c & d) FTIR and Raman Spectrum of (RuMOP-Pyz-1) respectively. |
|
Fig. 2 AFM 2D - Images of (a) Bare FTO; (b) Compact Layer of Nanocrystalline TiO2 (c) Active layer photoanode; (d) (RuMOP-Pyz-1) dye adsorbed on TiO2 active layer respectively. |
|
Fig. 3 FESEM Images of nanocrystalline TiO2 photoanode (a) Bare FTO; (b) Compact Layer; (c) Active layer; (d) Porosity on TiO2 active layer respectively. |
|
Fig. 4 (a) 2D AFM image and (b) FESEM image of platinum photocathode respectively. |
|
Fig. 5 Photovoltaic curve (J-V) measurements of fabricated DSSC device in which (RuMOP-Pyz-1) as sensitizer. (DARK - without sunlight, LIGHT - under Air Mass (AM) 1.5 G simulated sunlight at a light intensity of 100 mW/cm2) |
In summary, [Ru(II)(dcbpy)2(pyz)]n(BF4)2n
based metal organic polymer (RuMOP-Pyz-1) was
successfully synthesized and employed as a sensitizer in DSSC.
The synthesized metal organic polymer was characterized using
various spectroscopic and microscopic techniques. A maximum
conversion efficiency of 1.51% with short circuit current (Jsc)
3.22 mA/cm2; open circuit voltage (Voc) 0.684 V
and Fill Factor (FF) 68.45% under Air Mass (AM) 1.5 G simulated sunlight at
a light intensity of 100 mW/cm2 was obtained. The efficiency of the
device and its photovoltaic performances were found to be
satisfactory. The enhanced performance of the device is
attributed to the presence of extended conjugation of the metallo polymer which
helps in a facile electron transfer from the HOMO to LUMO of TiO2.
The results reported in this paper can provide new insights in
improving the performance of DSSC in future.
The authors wish to acknowledge the facilities and support
provided by PSG Sons & Charities Coimbatore, India.
- 1. B. O’ Regan and M. Gratzel, Nature 353 (1991) 737-740.
-

- 2. M.K. Nazeeruddin, R. Splivallo, P. Liska, P. Comte, and M. Grätzel, Chem. Commun. 12 (2003) 1456-1457.
-

- 3. M. Grätzel, Accounts. Chem. Res. 42 (2009) 1788-1798.
-

- 4. J.B. Baxter, J. Vac. Sci. Tech. A. 30 (2012) 020801.
-

- 5. A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, and H. Pettersson, Chem. Rev. 110 (2010) 6595-6663.
-

- 6. T.L. Bahers, E. Brémond, I. Ciofini, and C. Adamo, Phys. Chem. Chem. Phys. 16 (2014) 14435-14444.
-

- 7. A.S. Polo, M.K. Itokazu, and N.Y.M. Iha, Coordin. Chem. Rev. 248 (2004) 1343-1361.
-

- 8. A. Yella, H-W. Lee, H.N. Tsao, C. Yi, A.K. Chandiran, M.K. Nazeeruddin, E. Diau, C.Y. Yeh, S.M. Zakeeruddin, and M. Gratzel, Science 334 (2011) 629-634.
-

- 9. A. Carella, F. Borbone, and R. Centore, Front. Chem. 6 (2018) 481.
-

- 10. J. Albero and P. Atienzar, A. Corma, H. Garcia, Chem. Rec. 15 (2015) 803-828.
-

- 11. J.N. Clifford, E. Martínez-Ferrero, A. Viterisi, and E. Palomares, Chem. Soc. Rev. 40 (2011) 1635-1646.
-

- 12. L. Loh and S. Dunn, J. Nanosci. Nanotechno. 12 (2012) 1-16.
-

- 13. W. Wunderlich, T. Oekermann, L. Miao, N.T. Hue, S. Tanemura, and M. Tanemura, J. Ceram. Process. Res. 5 [4] (2004) 343-354.
- 14. C.-P. Lee, K.-M. Lee, P.-Y Chen, and K.-C. Ho, Sol. Energ. Mat. Sol. C. 93 (2009) 1411-1416.
-

- 15. H.S. Chen, S.J. Lue, Y.L. Tung, K.W. Cheng, F.Y. Huang, and K.C. Ho, J. Power. Sources. 196 (2011) 4162-4172.
-

- 16. Liu, Yalin Lu, Z.B. Xie, and G.M. Chow, Sol. Energ. Mat. Sol. C. 95 (2011) 800-803.
-

- 17. G. Monros, M. Llusar, A. García, C. Gargori, and R. Galindo. Adv. Sci. Tech. 68 (2010) 182-193.
-

- 18. S.I. Noh, T-Y. Seong, and H-J. Ahn, J. Ceram. Process. Res. 13 [4] (2012) 491-494.
- 19. H. An, G-H. An, and H-J. Ahn, J. Ceram. Process. Res. 16 [2] (2015) 208-212.
- 20. S.I. Noh and H-J. Ahn, J. Ceram. Process. Res. 13 (2012) s1-s5.
- 21. W-S. Kwack, H-J. Choi, W-C. Choi, H-R Oh, S -Y Shin, K. Moon, J-Y Kwak, Y-K. Jeong, S-H. Kwon. J. Ceram. Process. Res. 13[3] (2012) 338-342.
- 22. H. Lee, J-I. Park, T-H. Kim, and K-B. Park. J. Ceram. Process. Res. 14[3] (2013) 405-409.
- 23. F. Shao, J. Sun, L. Gao, S. Yang, J. Luo. J. Phys. Chem. C 115[5] (2011) 1819-1823
- 24. L. Shi, X. Liu, H. Li, and G. Xu. Anal. Chem. 78 (2006) 7330-7334.
-

- 25. S.Y. Tsai, C.T. Ni, and K.Z. Fung. Ceram. Int. 43 (2017) S460-S463.
-

- 26. S. Ahmada, A.K. Pandey, N.A. Rahima, S. Shahabuddinb, and S.K. Tyagi Ceram. Int. 44[15] (2018) 18444-18449.
-

- 27. M.I. Khana, M.A. Rehman, M. Saleem, M.R. Baig, S. Rehman, W.A. Farooq, M. Atif, and A. Hanif, Ceram. Int. 45[16] (2019) 20589-20592.
-

- 28. T. Oku, S. Nagaoka, A. Suzuki, K. Kikuchi, Y. Hayashi, H. Sakuragi, and T. Soga, J. Ceram. Process. Res. 9[6] (2008) 549-552.
- 29. Y. Ren, D. Sun, Y. Cao, H.N. Tsao, Y. Yuan, S.M. Zakeeruddin, P. Wang, and M. Gratzel, J. Am. Chem. Soc. 140[7] (2018) 2405-2408.
-

- 30. N.A. Ludin, A.M. Mahmoud, A.B. Mohamad, A.A.H. Kadhum, K. Sopian, and N.S. Abdul Karim, Renew. Sust. Energ. Rev. 31 (2014) 386-396.
-

- 31. Y.S. Yen, H.H. Chou, Y.C. Chen, C.Y. Hsu, and J.T. Lin, J. Mater. Chem. 22 (2012) 8734-8747.
-

- 32. W.Y. Wong, J. Organomet. Chem. 694 (2009) 2644-2647.
-

- 33. N. Kakuta, T. Oku, A. Suzuki, K. Kikuchi, and S. Kikuchi, J. Ceram. Process. Res. 13[1] (2012) 28-31.
- 34. C.A. Bignozzi, R. Argazzi, R. Boaretto, E. Busatto, S. Carli, F. Ronconi, and S. Caramori, Coordin. Chem. Rev. 257 (2013) 1472-1492.
-

- 35. S. Sethi, S. Jena, P.K. Das, and N. Behera, J. Mol. Struc. 1193 (2019) 495-521.
-

- 36. S.S. Mali, C.A. Betty, P.N. Bhosale, and P.S. Patil, Electrochim. Acta. 59 (2012) 113-120.
-

- 37. C. Cai, S. Tseng, M. Kuo, K. Lin, H. Yang, and R. Lee RSC. Adv. 5 (2015) 102803-102810.
-

- 38. B. Nagarajan, S. Kushwaha, R. Elumalai, S. Mandal, K. Ramanujam, and D. Raghavachari, J. Mater. Chem. A. 5 (2017) 10289-10300.
-

- 39. T. Daeneke, T.H. Kwon, A. B. Holmes, N. W. Duffy, U. Bach, and L. Spiccia, Nat. Chem. 3 (2011) 211-215.
-

- 40. Q. Jiang, Z. Chu, P. Wang, X. Yang, H. Liu, Y. Wang, and J. You, Adv. Mater. 29 (2017) 1703852.
-

- 41. B. Hailegnaw, S. Kirmayer, E. Edri, G. Hodes, and D. Cahen, J. Phys. Chem. Lett. 6 (2015) 1543-1547.
-

- 42. P. Billen, E. Leccisi, S. Dastidar, S. Li, L. Lobaton, S. Spatari, and J. B. Baxter, Energy, 166 (2018) 1089-1096.
-

- 43. S. Yun, Y. Qin, A.R. Uhl, N. Vlachopoulos, M. Yin, D. Li, and A. Hagfeldt, Energy Environ. Sci. 11 (2018) 476-526.
-

- 44. R. Su, S. Ashraf, and A. El-Shafei, Sol. Energy 177 (2019) 724-736.
-

- 45. J.A. Cuello-Garibo, C.C. James, M.A. Siegler, and S. Bonnet, Eur. J. Inorg. Chem. 25 (2018) 1260-1268.
-

- 46. J.C. Salsman, S. Ronco, C.H. Londergan, and C.P. Kubiak, Inorg. Chem. 45 (2006) 547-554.
-

- 47. Q. Yu, S. Liu, M. Zhang, N. Cai, Y. Wang, and P. Wang, J. Phys. Chem. C 113 (2009) 14559-14566.
-

- 48. D. Bari, N. Wrachien, R. Tagliaferro, S. Penna, T.M. Brown, and A. Reale, A. Cester, Microelectron. Reliab. 51 (2011) 1762-1766.
-

- 49. A. Wild, A. Winter, and F. Schlütter, U. S. Schubert, Chem. Soc. Rev. 40 (2011) 1459-1511.
-

- 50. K. Feng, X. Shen, Y. Li, Y. He, D. Huang, and Q. Peng, Polym. Chem. 4 (2013) 5701-5710.
-

- 51. J. Xiang, C.L. Ho, and W.Y. Wong, Polym. Chem. 6 (2015) 6905-6930.
-

- 52. A.L.A. Parussulo, T.A. Matias, R.R. Guimaraes, S.H. Toma, K. Araki, and H.E. Toma, Inorg. Chim. Acta, 453 (2016) 764-770.
-

- 53. A.M.W.C. Thompson, M.C.C. Smailes, J.C. Jeffery, and M.D.J. Ward, J. Chem. Soc. Dalton Trans. (1997) 737-744.
-

- 54. M. Plevoets, F. Vögtle, L. De Cola, and V. Balzani, New J. Chem. 23 (1999) 63-69.
-

- 55. M.K. Nazeeruddin, S.M. Zakeeruddin R.H. Baker, M. Jirousek, P. Liska, N. Vlachopoulos, V. Shklover, C-H. Fischer, and M. Gratzel, Inorg. Chem. 38[26] (1999) 6298-6305.
-

- 56. C.A. Mitsopoulou, I. Veroni, A.I. Philippopoulos, and P. Falaras, J. Photoch. Photobio. A. 191 (2007) 6-12.
-

 This Article
This Article
-
2020; 21(1): 123-130
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.123
- Received on Dec 9, 2019
- Revised on Dec 15, 2019
- Accepted on Dec 24, 2019
 Services
Services
- Abstract
introduction
experimental section
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Sivasubramanian Ramanathan
-
Electrochemical Energy Materials and Sensors Laboratory, NRIIC, PSG Institute of Advanced Studies, Coimbatore-4, Tamilnadu, India
Tel : +422 434 4000 Fax: +422 257 3833 - E-mail: rss@psgias.ac.in







 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.