- Low temperature sintering of Al2O3 according to the amount of Bi2O3 additive considering economics
Ye-Na Leea, Hoseok Namb,* and Ki-Woo Nama
aDepartment of Materials Science and Engineering, Pukyong National University, Busan 48547, Korea
bInstitute of Economic Research, Kyoto University, Yoshida-honmachi, Sakyo, Kyoto, 606-8501, Japan
Structural ceramics are
generally superior in strength to metal at high temperatures. However, they
must be sintered at high temperatures to achieve this high strength. Therefore,
lowering the sintering temperature would have economic benefits, including in
terms of energy consumption. This study investigated the possibility of
reducing the sintering temperature by adding bismuth oxide(Bi2O3)
to the sintering of aluminium oxide(Al2O3). The amount of
Bi2O3 additive was changed to 12~19.1 wt.% and the
sintering temperature was in the range of 850~1200 oC, and the
compressive strength was evaluated under these conditions. The addition of Bi2O3
can decrease the necessary sintering temperature by about 400 oC.
Ceramic structures that can be low temperature sintering can save costs due to
reduced manufacturing costs, replacement costs, shutdowns, etc., which will
ensure enormous economic efficiency.
Keywords: Al2O3, Bi2O3, Sintering temperature, Compressive strength, Economic effect
Aluminium oxide (Al2O3) is an advanced ceramic material that is used in a wide variety of
applications due to its excellent fire resistance, chemical stability, and wear
and deformation resistance. It is also a relatively abundant and low-cost
resource, making it particularly attractive for commercial applications. Al2O3, like other ceramics, has excellent high temperature stability and high
temperature strength. Al2O3 is known to have a direct effect on the sintering
process because it changes the microstructure and interfacial energy of the
sintered material with the use of additives. Silicon oxide (SiO2) suppresses densification but
promotes grain growth [1]. Magnesium oxide
(MgO) can suppress the abnormal grain growth of Al2O3, and it can densify it to near theoretical density [2, 3]. The
addition of titanium
oxide(TiO2) promotes not only the densification of Al2O3 but also grain growth [4, 5]. meanwhile, the addition of yttrium oxide(Y2O3) increases the strength and sintering properties [6-11]. However, the
authors was sintered Al2O3 at 1,600 oC and studied it [6]. Ando et al was sintered Mullite/SiC by
hot-pressing at 1,650 oC [11]. The addition of manganese oxide (MnO) has been shown to increase the density and lower the sintering temperature
[12-14]. Bi2O3 has a low melting point of 825 oC, and is therefore considered to
be a suitable material for use as a ceramic sintering additive. De Marco V. et
al. reduced the normal sintering temperature by more than 400 oC by using Bi2O3 as an additive of Gd: cerium oxide (CeO2). [15] In another study, barium zirconate (BaZrO3) was contracted by 19.0% at
1,480 oC with the use of 3 mol% Bi2O3 additives, and the mixture reduced pore volume at sintering for 24
hours of 1,400 oC [16].
This study investigated the effect of using sintering additive Bi2O3 on compressive strength by varying the
amount of Bi2O3 as well as the sintering temperature in the sintering of Al2O3. From this study, the addition of Bi2O3 can lower the sintering temperature of alumina by about 400 oC.
The Al2O3 powder used in this
experiment had an average particle diameter of 0.5 µm (Korea CIS Co., Ltd.),
the SiC powder had an average particle diameter of 0.27 µm (Betarundum UF,
Ibiden, Japan), and the Y2O3 powder was 0.27 µm fine
grade from Nippon Yttrium. Bi2O3 was obtained from
Daejung chemicals & metals Co., Ltd (Korea).
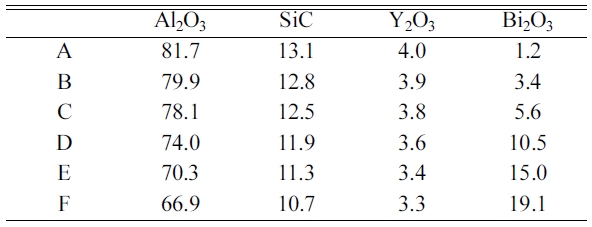
The mixing ratio of the powder is
shown in Table 1. A mixture of Al2O3, SiC, Y2O3, and Bi2O3 was mixed for 24 hours along with Al2O3
balls and alcohol. The mixed powder was then dried
in a 100 oC. furnace (Jisco,
Model: J-300M) for 3 h before being filtered in a 100 μm sieve. next, the dry powder was placed into a cylindrical mold with a
diameter of 10mm and molded to a pressure of 30 MPa.
atmospheric sintering proceeded in a tubular furnace (Lenton, Model: LTF-180) of 1,200 oC for 1 h and 30 min. The
temperature increased at a rate of 5 oC/min. The properties of the sintering material were determined based on
compressive strength and density. The fracture surface of the sintering
material was observed by SEM (Scanning Electron Microscope: Hitachi (Japan), S-2700). The components of the sintering material were analyzed by EDX
(Energy Dispersive X-Ray Spectrometer: Horiba (Japan)).
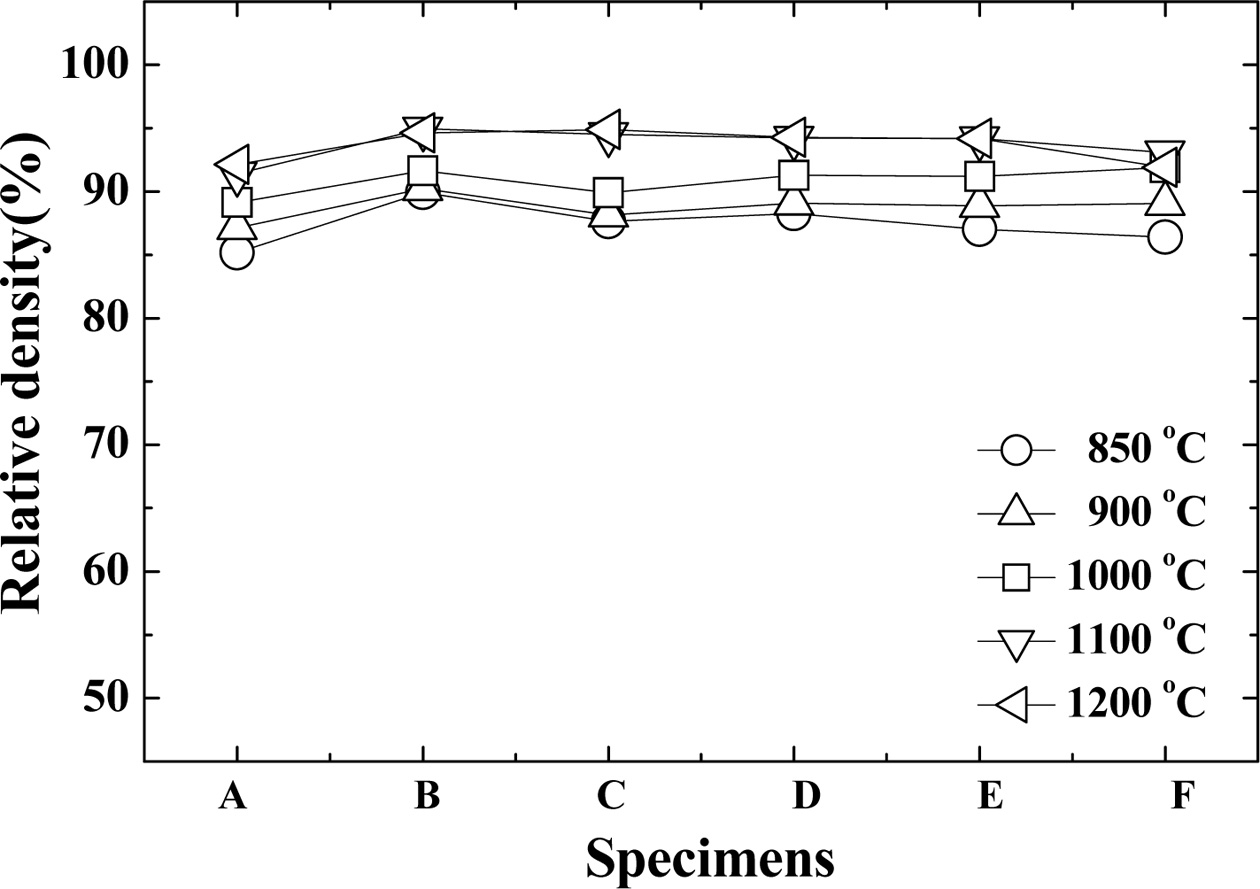
Fig. 1 shows the relative density of the Al2O3 sintering materials according to the amount
of Bi2O3 added. This represents the percentage of Al2O3 theoretical density. The theoretical density of Al2O3 is 3.95 g/cm3. It can be seen that relative density increases as the sintering
temperature is increased when the amount of Bi2O3 is constant. In addition, at a low sintering temperature of 850 °C,
the “A” specimen containing the least amount of Bi2O3 showed a relative density of about 85% while the “E” specimen
containing the highest amount of Bi2O3 showed a relative density of about 86%. The
relative densities of the “B~E” specimens showed almost similar relative
densities at each temperature. The relative density of the “B” specimen at
1,100 oC was the highest at about 94.9%. The relative density of the specimen
sintered at 1,200 oC was slightly lower than that of 1,100 oC. The Al2O3 sintering material with Bi2O3 additive shows excellent relative density when the sintering
temperature is 1,100 oC and 1,200 oC, and its compressive strength is high. However, the “A” specimen with low Bi2O3 content and the “E” and “F” specimens with high Bi2O3 contents were small at 850 oC and 1,200 oC, and the relative density also
decreased.
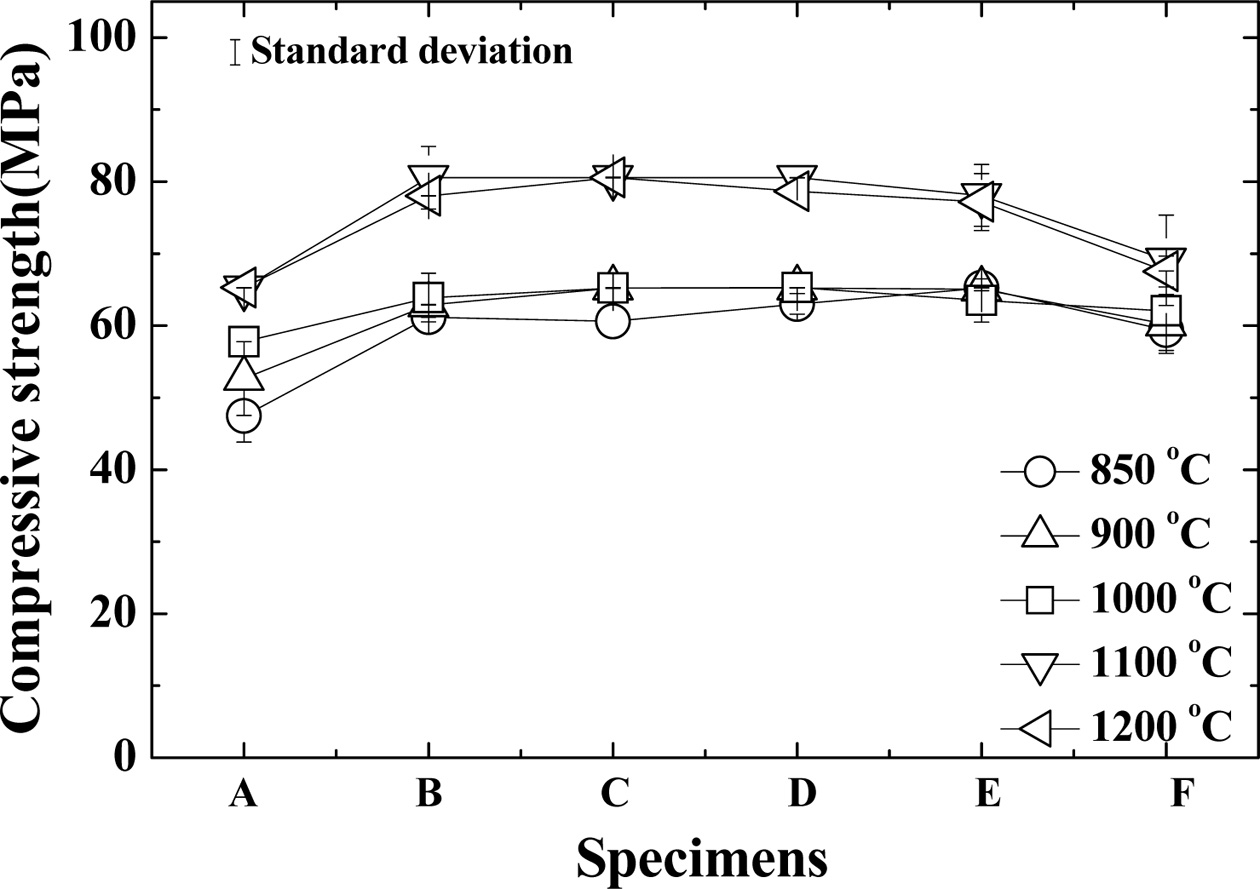
Fig. 2 shows the compressive
strength of the Al2O3 sintering material according to the amount of Bi2O3
added. The figure also shows the standard deviation. The compressive
strength was divided into two groups regardless of the amount of Bi2O3 added: 1,000 oC or less
and 1,100 oC or more. In the case of “A” specimens with a small amount of Bi2O3, the compressive strength increased as the sintering temperature
increased. The compressive strengths of the “B~E” specimens showed almost the
same compressive strength at each sintering temperature. In the “F” specimen, there was a slight decrease in compressive strength. The
compressive strength of the “B” specimen was the highest at 1,100 oC and 1,200 oC, but its compressive strength
decreased as the content of Bi2O3 increased. This indicates that the addition of Bi2O3 can lead to a decrease of about 400~500 oC compared to the sintering
temperature of 1,600 oC of Al2O3 with Y2O3 additive [17-20]. This is because the sintering material became dense
as the sintering temperature increased.
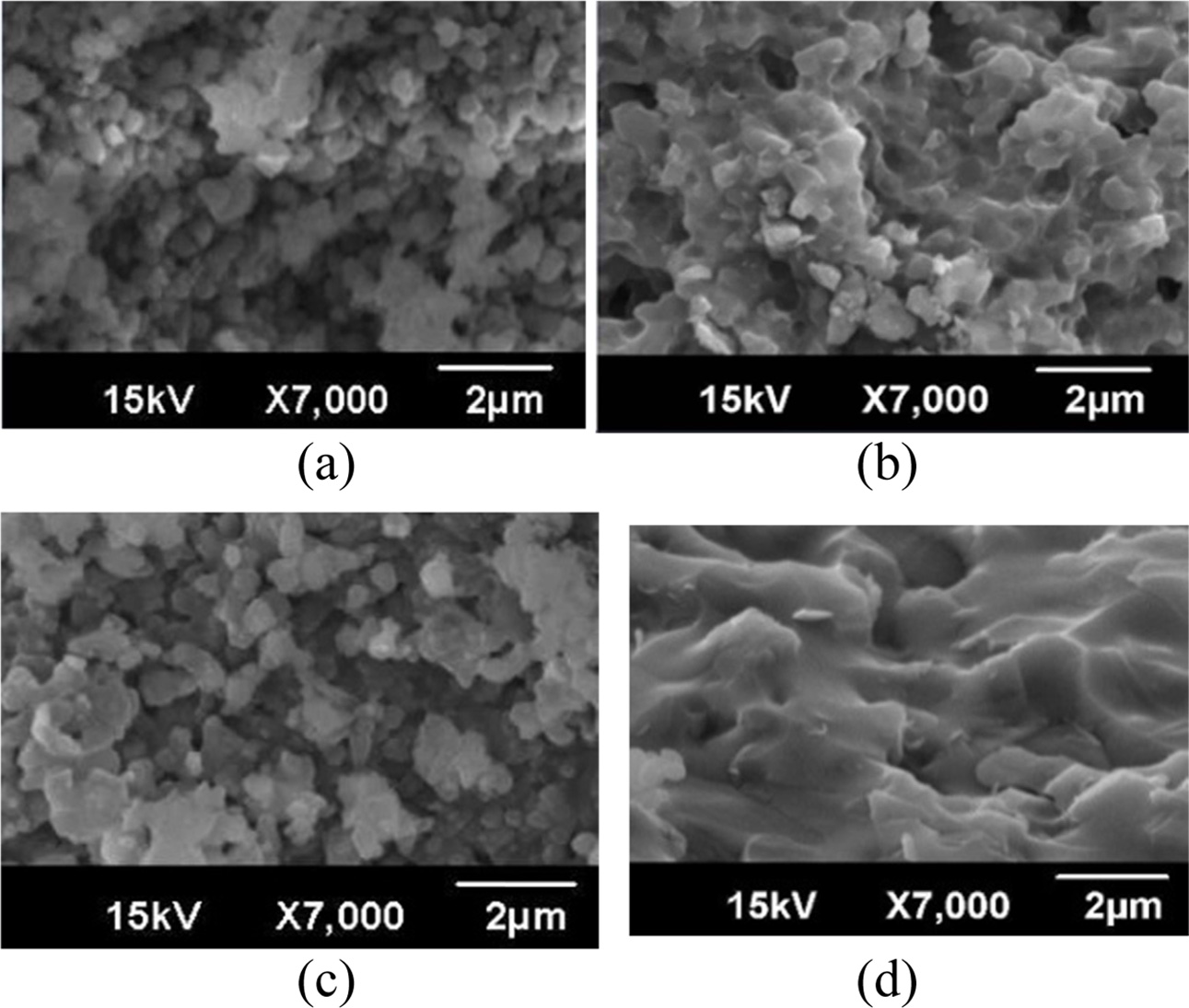
Fig. 3 shows the SEM results of the fracture surface. (a) shows a “B” specimen sintered at 900 oC and (b) shows a “B” specimen
sintered at 1,100 oC. (c) and (d) are “A” and “F” specimens sintered at 1,200 oC. When the same amount of Bi2O3 was added, it was confirmed that the relative density and the
compressive strength increased as the sintering temperature increased. At each sintering temperature, the “A”
and the “F” specimens appeared small while the “B~E” specimens appeared to be almost similar. (a) confirms that sintering was not completed because the
temperature was low, and as a result the particles were not sintered. (b) shows
the best condition of relative density and compressive strength. This means
that the particles are sintered, and that many micro pores formed
between the particles. Many of these pores disturb crack propagation, leading
to it having the highest compressive strength. Although (c) had a sintering
material of 1,200 oC, the fracture surface was similar to the result
of (a). (d) shows that Bi2O3 melted and penetrated between particles due to the large amount of Bi2O3, resulting in low sintering power between particles. As the
brittleness is strong and cracks rapidly propagate, the compressive strength is
considered to be reduced.
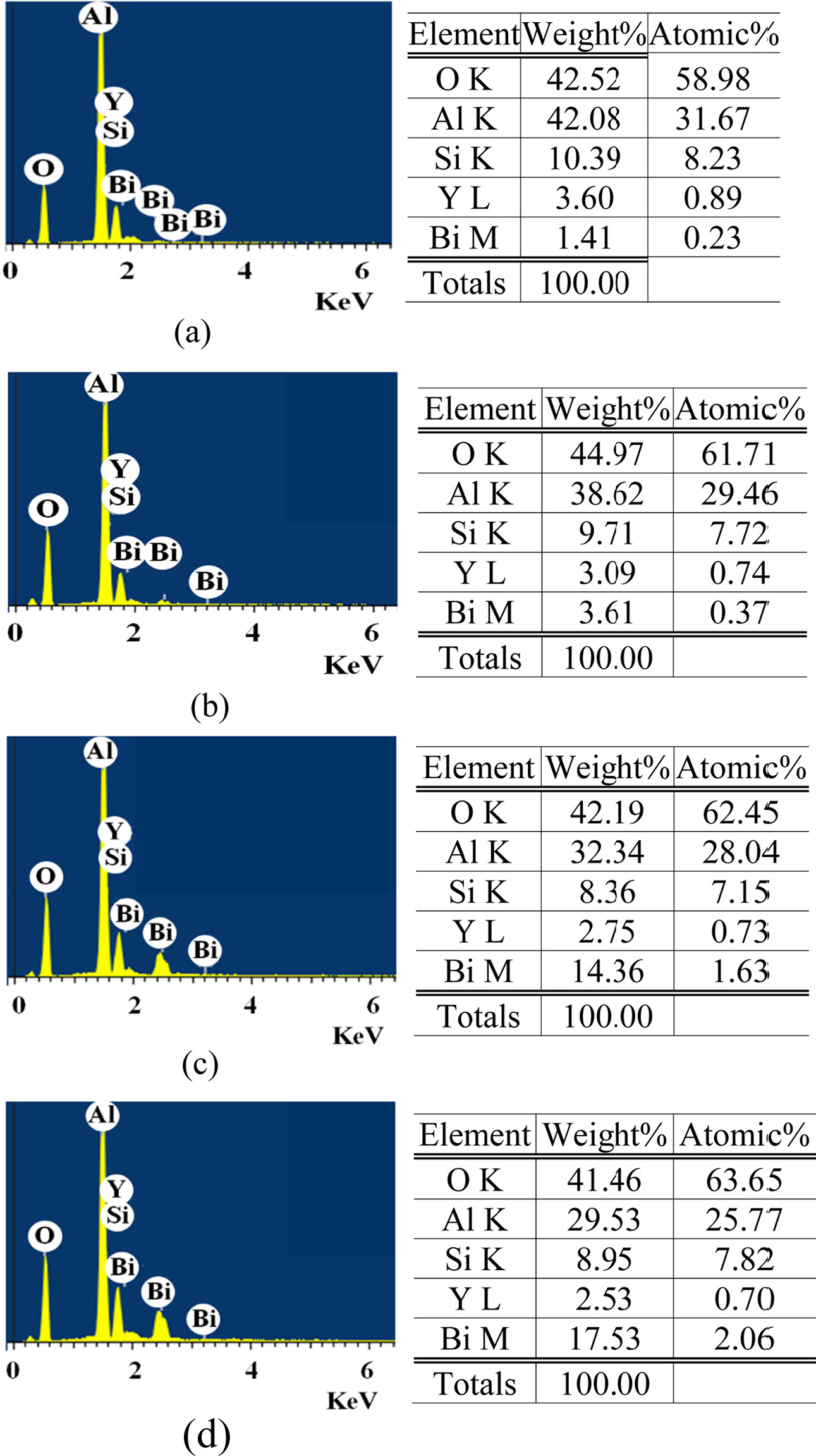
Fig. 4 shows the EDX analysis
result of the fracture surface. (a) and (b) show “B” specimens sintered at
900 oC and 1,100 oC, respectively, and (c) and (d) respectively show “D” specimens and
“F” specimens at 1,200 oC. (a) and (b) show “B” specimens with the same amount of Bi2O3. More Bi2O3 was detected at 1,100 oC than 900 oC. This is because Bi2O3 melts and covers the surface of the sintered ceramic particles.
Naturally, the sintering material of high temperature detected a lot of oxygen.
(c) and (d) were sintered at the same temperature, but the amount of Bi2O3 was different. (c) indicates that it is subject to the same factors as
(b), but since (d) measured the quantity of the surface, it is judged that not
all of the addition amount was detected. Therefore, the results shown in Figs. 1 to 3 indicate that the addition of Bi2O3 can lower the sintering temperature, but the optimal amount of
additive is considered to be around 3.4 wt.%.
This study proved the lower sintering temperature at 1,100
oC or 1,200 oC can achieve similar performance by
adding Bi2O3 to Al2O3 compared with
Al2O3 sintered at 1,600 oC in the
previous study [6]. By decreasing the sintering temperature, it is possible to
reduce the time for sintering which lead to energy saving. It was calculated
that the sintering temperature at 1,100 oC or 1,200 oC
can save energy use by 9.1% and 4.5% respectively compared with that of 1,600 oC.
|
Fig. 1 Relative density of the sintered Al2O3 according to the amount of Bi2O3. |
|
Fig. 2 Compressive strength of the sintered Al2O3 according to the amount of Bi2O3. |
|
Fig. 3 SEM observation of fracture surface. (a) “B” specimen sintered at 900 oC, (b) “B” specimen sintered at 1,100 oC, (c) “A” specimen sintered at 1,200 oC, and (d) “F” specimen sintered at 1,200 oC. |
|
Fig. 4 EDX analysis of fracture surface. (a) “B” specimen sintered at 900 oC, (b) “B” specimen sintered at 1,100 oC, (c) “D” specimen sintered at 1,200 oC, and (d) “F” specimen sintered at 1,200 oC. |
In this study, Bi2O3 was added to the sintering of Al2O3 ceramics to evaluate the sintering characteristics according to the
addition amount. The obtained results are as follows.
As the sintering temperature increases, the compressive strength and relative density of
the sintering material tend to increase, but the compressive strength is decreased when more than 15 wt.% of Bi2O3
is added.
The specimens containing more than
15 wt.% of Bi2O3 shows decreased compressive strength and relative density when sintered at 1,200 oC.
It is judged that the compressive
strength of 1,100 oC and 1,200 oC is excellent by adding 3.4~10.5 wt.% of Bi2O3. The optimal condition was
strictly obtained from 3.4 wt.% of Bi2O3 at 1,100 oC. The addition of Bi2O3 can lower the sintering temperature of alumina by about 400 oC.
Sintering process of Bi2O3-added Al2O3 at 1,100 oC and 1,200 oC can reduced energy use by 9.1% and 4.5% respectively compared with that
of sintered Al2O3 at 1,600 oC. Ceramic structures that can be
low temperature sintering can save costs due to reduced manufacturing costs,
replacement costs, shutdowns, etc., which will ensure enormous economic
efficiency.
- 1. P.C. Harola and J.C. Carl, J. Am. Ceram. Soc. 39[10] (1956) 337-344.
-

- 2. A.H.De Aza, J.E. Iglesias, P. Pena, and S.D. Aza, J. Am. Ceram. Soc. 83[4] (2004) 919-927.
-

- 3. T. Durán, S. Serena, P. Pena, and A. Caballero, J. Am. Ceram. Soc. 91[2] (2008) 535-543.
-

- 4. T. Ikegami, K. Kotani, and K. Eguchi, J. Am. Ceram. Soc. 70[12] (1987) 885-890.
-

- 5. R.D. Bagley, D.L. Johnson, and I.B. Cuttler, J. Am. Ceram. Soc. 53[3] (1970) 136-141.
-

- 6. H.S. Kim, M.K. Kim, J.W. Kim, S.W. Ahn, and K.W. Nam, Trans. KSME A 31[4] (2007) 425-431.
-

- 7. T. Mitamura, H. Kobayashi, N. Ishibashi, and T. Akiba, J. Ceram. Soc. Jpn. 99[1149] (1991) 351-356.
-

- 8. C.S. Hwang and D.Y. Fang, J. Ceram. Soc. Jpn. 100[1165] (1992) 1159-1164.
-

- 9. D.Y. Fang and C.S. Hwang, J. Ceram. Soc. Jpn. 101[1171] (1993) 331-335.
-

- 10. K. Ando, K. Tsuji, M. Nakatani, M.C. Chu, S. Sato, and Y. Kobayashi, J. Soc. Mater. Sci. Jpn. 51[4] (2002) 458-464.
- 11. J. She, P. Mechnich, M. Schmucker, and H. Schneider, J. Eur. Ceram. Soc. 22[3] (2002) 323-328.
-

- 12. X.L. Yin, L. Liu, X. Shen, M.L. He, L. Xu, N. Wang, and M. Chen, EPD Congress (2016) 119-124.
-

- 13. Y. Taniguchi, N. Sano, and S. Seetharaman, ISIJ International 49[2] (2009) 156-163.
-

- 14. M. Sathiyakumar and F.D. Gnanam, Ceramics International 28[2] (2002) 195-200.
-

- 15. V. De Marco and V.M. Sglavo, ECS Trans. 68[1] (2015) 413-420.
-

- 16. S. Le, J. Zhang, X. Zhu, J. Zhai, and K. Sun, J. Power Sources 232 (2013) 219-223.
-

- 17. K.W. Nam, H.S. Kim, C.S. Son, S.K. Kim, and S.H. Ahn, Trans. KSME A 31[11] (2007) 1108-1114.
-

- 18. K.W. Nam, J. Ceram. Process. Res. 11[4] (2010) 471-474.
- 19. S.H. Ahn and K.W. Nam, J. Ceram. Process. Res. 18[9] (2017) 646-658.
- 20. K.H. Lee and K.W. Nam, J. Ceram. Process. Res. 19[1] (2018) 75-79.
 This Article
This Article
-
2020; 21(1): 99-102
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.99
- Received on Oct 10, 2019
- Revised on Dec 18, 2019
- Accepted on Dec 24, 2019
 Services
Services
- Abstract
introduction
materials and experiment methods
results and discussion
conclusions
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hoseok Nam
-
Institute of Economic Research, Kyoto University, Yoshida-honmachi, Sakyo, Kyoto, 606-8501, Japan
Tel : +81-75-753-7175 Fax: +81-75-753-7178 - E-mail: namhs0107@gmail.com






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.