- Comprehensively utilizing waste coal gangue to fabricate high strength glass-ceramics
W. Danga and H.-Y. Heb,*
aXi'an Aeronautical University, Xi'an 710077, China
bCollege of Material Science and Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China
Waste coal gangue was utilized for the fabrication of strength glass-ceramics for
environmental management. The optimal utilization rate of
coal gangue is 55 wt.% when the utilization rate of another mineral material bauxite is 35 wt.%. The mullite showing high strength is the main crystal
phase in the sintered glass-ceramics. The effect of various processing
parameters on the microstructure and mechanical performances of the sintered
glass-ceramics was systematically studied. Mineralization agent BaCO3
and MnCO3 resulted in remarkable enhancement of the mechanical
properties of the sintered glass-ceramics. The strength of 148.36-156.12 MPa, water absorption of 0.17-0.02%, and a
density of 2.66-2.68 g/cm3 were achieved for the sintered glass-ceramics. The process of forming and sintering also showed a remarkable
effect on the microstructural and mechanical properties. By optimization experiments,
optimal processing parameters were also determined.
Keywords: Coal gangue; Glass-ceramics, Waste management, Maximal utilization ratio, Process optimization, Mechanics property
Glass-ceramics are attractive materials used in various applications
such as building materials, cooking ceramics, machinable
ceramics, bio-ceramics, electrical ceramics, glass semiconductors in thermal
insulation, optical materials, etc. [1-6]. From a point of the raw material,
utilizing the industrial solid wastes were widely used to fabricate the g;ass-ceramics and cecrents
et al. [7-10].
Coal gangue is a category of solid industrial waste. This
waste material is largely produced in coal production. The amount
produced in China is reached above several million tonnes and increases by
about one million tonnes per year. To prevent its pollution to air and river
environments and save natural resource, the comprehensively utilizing the coal
gangue are urgent for efficient environmental management.
However, the ratio of utilizing coal gangue is greatly limited. The coal gangue contains a
high alumina of ~30 wt.% and a high silica of ~62
wt.%, and so has been used to produce cement [11-13], cement [14], ceramics
[15, 16], glass-ceramics [17, 18], brick [19, 20], zeolite [21], lightweight
aggregate [22], and so on. The glass-ceramics mainly contains the crystals and
amorphous phase that is composed of SiO2, Al2O3,
CaO, and/or MgO. The mullite and some other crystals composed of both alumina
and silica are favorable for the high strength of the glass-ceramics. The high
content of SiO2 and Al2O3 makes the coal
gangue is very suitable to fabricate the glass-ceramics having high mechanical
properties.
For sintering high strength glass-ceramics, the crystal
phase and its size and content are critical factors. In general, mullite and
other crystals composed of both aluminium and silicon will be favorable for the
high strength of the glass-ceramics. Meanwhile, the nucleating agent
and some process parameters are the deterministic factors for
the size and content of the formed crystal phase as well as the density of the
sintered glass-ceramics.
This work focuses on the fabricating the glass-ceramics by
utilizing waste material coal gangue and the optimizing coal gangue utilization
ratio, nucleating agent, and processing parameters. The glass-ceramics with the mullite as main crystal phase were successfully obtained by selecting the
reasonable prescriptions of the glass-ceramics in the
condition of considering the maximal utilization rate of
the coal gangue. The optimization of nucleating agent and process parameter
were also focused to further realize the enhancement of property of the
sintered glass-ceramics.
Mullite (Al6Si2O13) generally has high strength and so was designed as the main crystal phase. The mullite (Al6Si2O13) contain higher alumina than the
coal gangue (Table 1). Thus, the bauxite
with high alumina content was utilized to be in favor of the formation of
mullite crystal phase. Clay as an adhesive was utilized to enhance slurry formability. Besides,
small amounts of steatite and limestone were used as
additives; and BaCO3, MnCO3, and MgCO3
were used as mineralizers. The sodium
tripolyphosphate (STPP) and methylcellulose
(CMC) as water reducers were utilized to
decrease the slurry
viscosity. The chemical composition of the bauxite and clay together with coal gangue
and some mineral minerallizers is listed in Table 1. In consideration of the utilization ratio of the coal gangue, the basic
prescriptions were designed
as listed in Table 2.
Typical sintering processes of the
designed glass-ceramics are mentioned below: the raw materials were first mixed
according to the designed prescriptions. The mixtures were filtered through 10 mesh,
wet-milled at the ratio of raw materials : ball : water
= 1 : 2.5 : (0.3-0.5) for 15-25 min and then filtered through 120 mesh (Aperture: 0.045 mm, ASTM). As prepared mixtures were undergone a 3-5 h drying of 50-70 oC. The 200 g
each of the dried mixtures was mixed with 5 g STPP and 5 g CMC and ball-milled using the appropriate
amount of water for 12 min. The formed slurries were sifted
with a serve (80 mesh, aperture: 0.180 mm, ASTM). The ~0.1% residue above the
sieve only remained. The slurries were dried at 50-70 oC for 3-5 h. The formed powders
underwent a pulverization and then sifted with a sieve (55 mesh, aperture: 0.280 mm, ASTM). The fine powders were sprayed by
water and sintered to form prilled grains. The prilled grains were sifted with
a sieve (20 mesh, aperture: 0.850 mm, ASTM). The fine grains were undergone
re-spraying water and then processed to be the samples for the measurement of flexural strength. The formed
samples were then pressed at 25-35 MPa and dried at 50-100 oC for 24 h. The large grains on the
sieve were dried at 80 oC for 4 h. The dried samples and large grains were finally sintered at 1290-1430 oC for 30-90 min at a heating rate of 5-10 oC/ min.
The sintered glass-ceramics samples were characterized with
D/Max-2200PC X-ray diffractometer (XRD, CuKα1, λ=0.15406
nm), S-570 scanning electron microscopy (SEM), and the flexural strength measurement on 401/3
strength tester. The water absorbance (Aw) was measured
on the sintered grain samples. On the measured Wd and Wh,
the Aw was specified by the following relation:
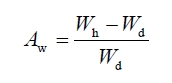
In which, Wd
is the weight of some grains that undergone the drying at 110 oC
for 3 h, Wh is the weight of the above-used grains undergone
the soaking in the water for 36 h at room temperature followed by erasing the
surface water with a wet towel. The density (d) was measured on the
sintered grain samples, and specified with the following relation:
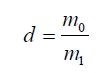
Where m0 is the mass of some grain
samples that undergone the 3 h drying at 105-110 oC, m1 is the mass of the above-used grain
samples when immersed in the water at room temperature. The withdrawn grains underwent the erasion of the surface water with a wet towel.
All the property measurements were
repeated three times to obtain accurate results.
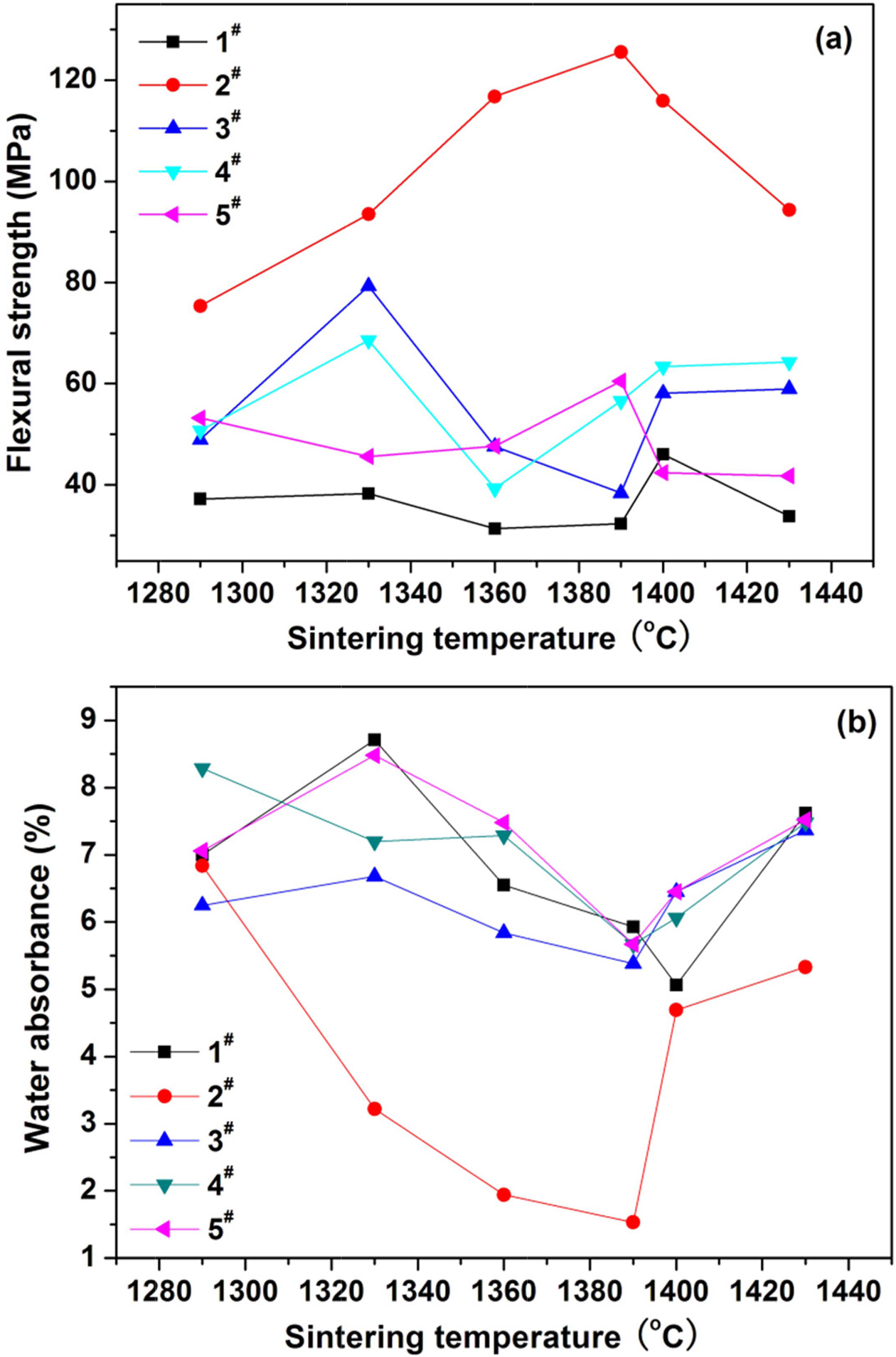
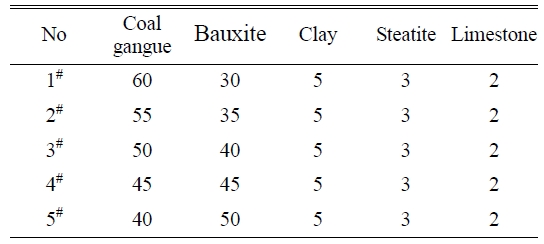
The five samples with the basic
prescriptions listed in Table 2 were first tested to determine
optimal utilization ratio of the coal gangue. Fig. 1 shows the variations of flexural strength and water
absorbance of glass-ceramics fabricated with basic prescriptions as a function of sintering temperature. The
glass-ceramics fabricated with prescription 2# shown a maximal strength and minimal water
absorbance in the sintering temperature range of 1290-1430 oC. Therefore, the utilization ratio
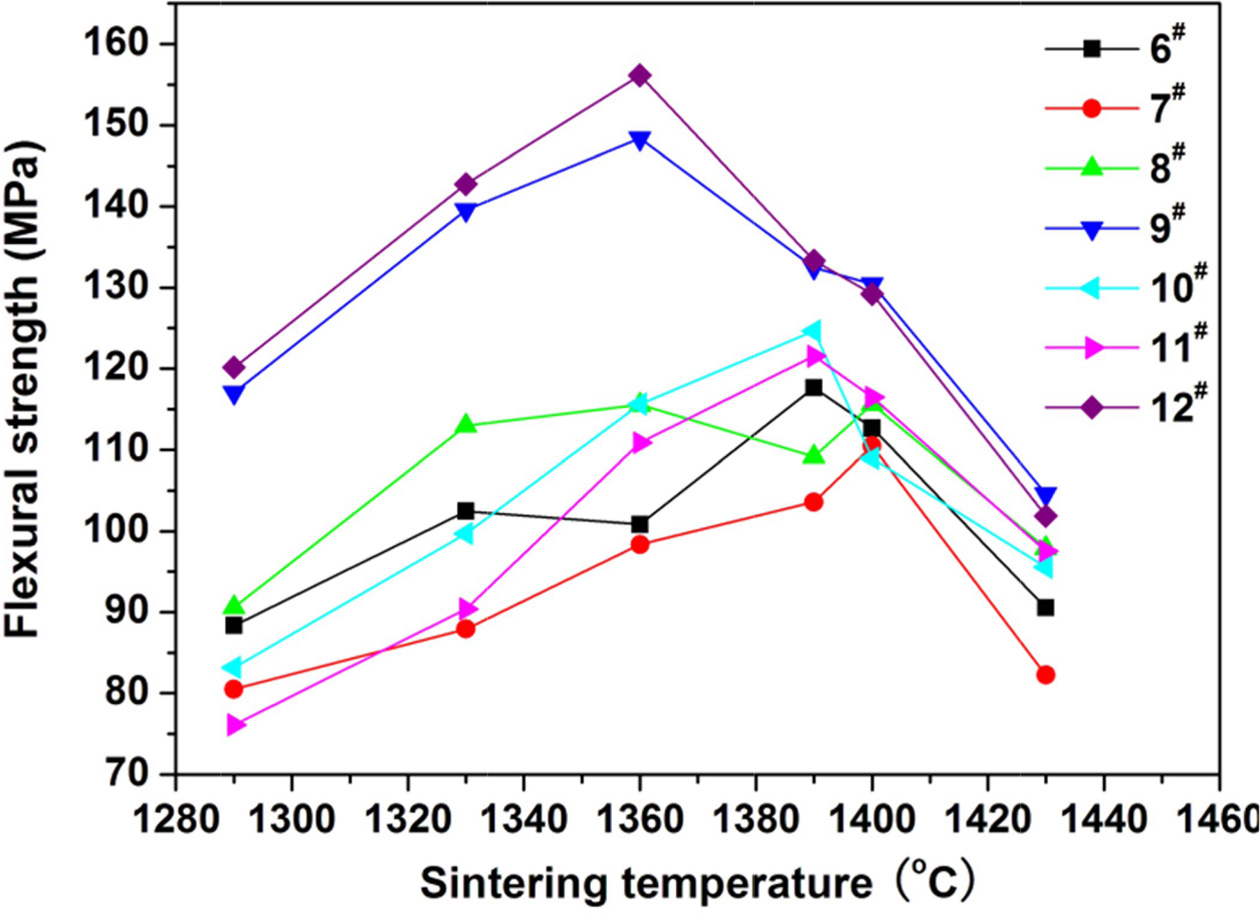
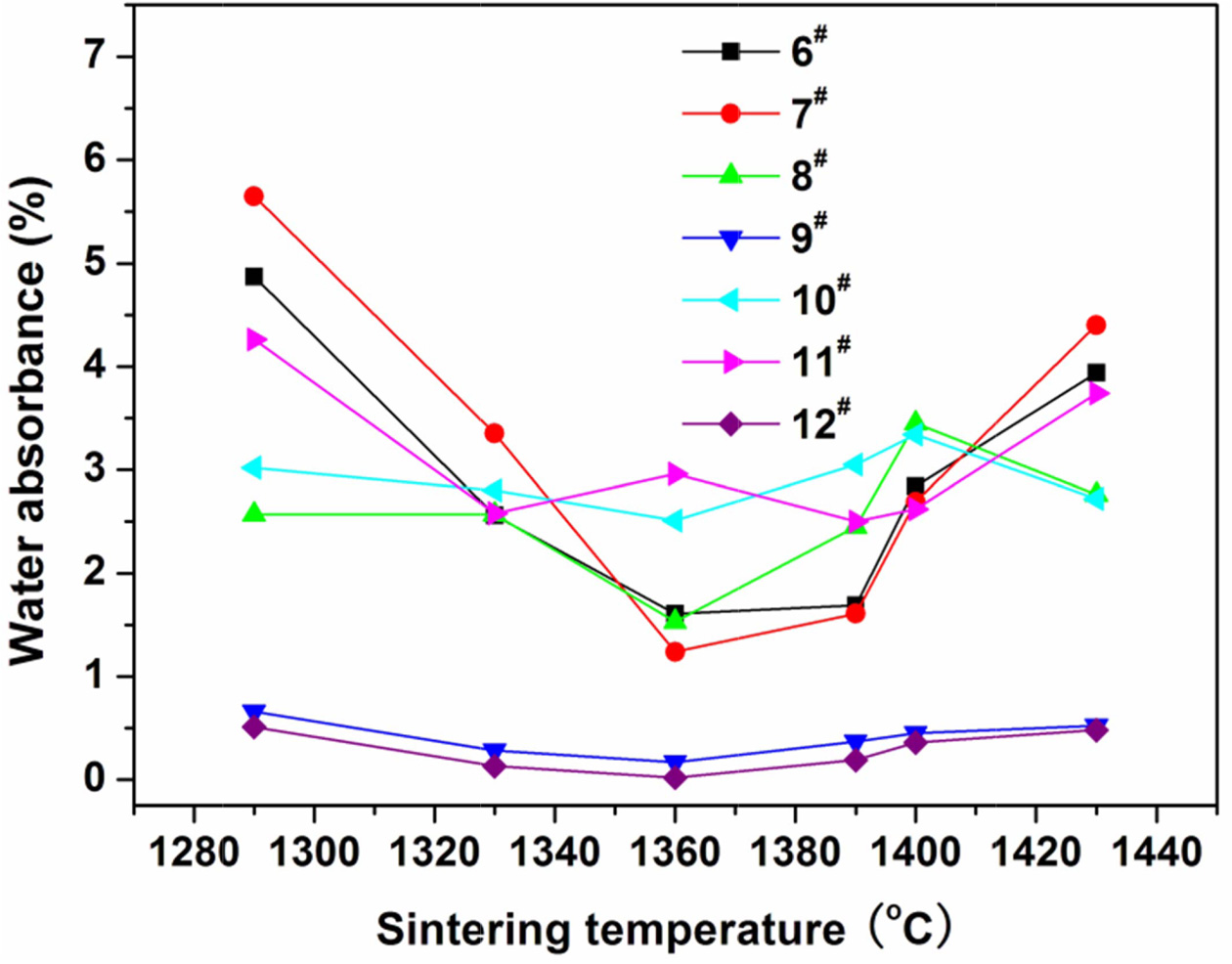
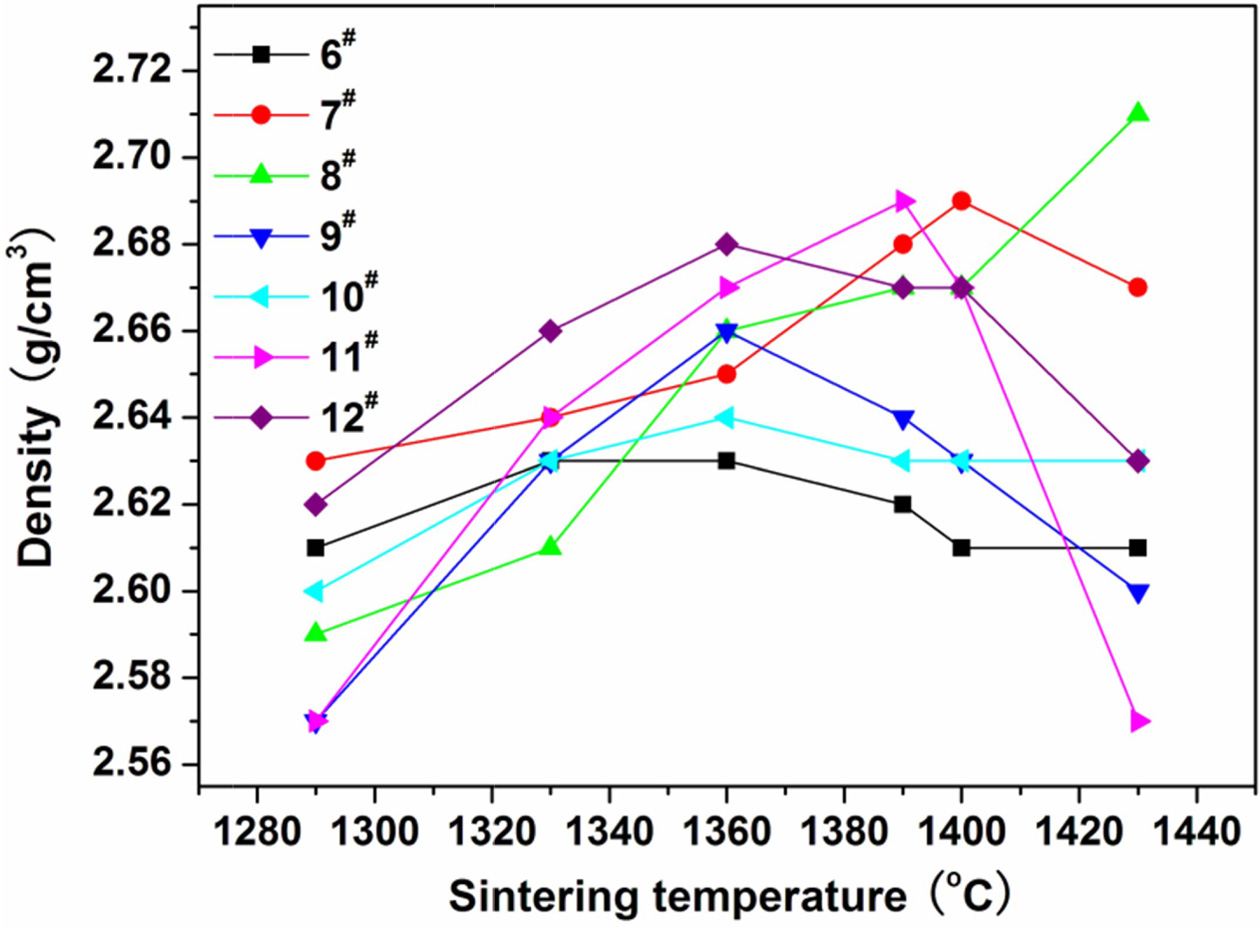
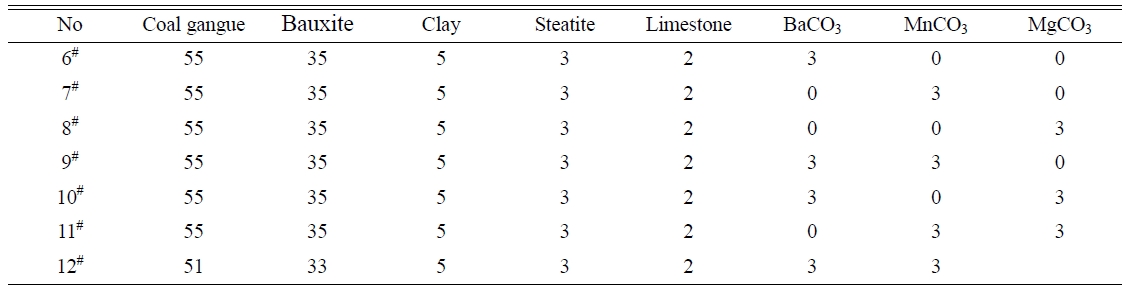
of 55% is determined for the coal gangue. The prescription 2# was further studied by adding various mineralizers. Table 3 lists the new prescriptions. Fig. 2, 3, and 4 illustrate the properties of the glass-ceramics
formed at the
press of 30 MPa and sintered at different temperatures for 30 min. BaCO3
and MnCO3 co-adding (prescription
9#) lead to better effect, achieving
a minimal water absorption (0.17%), maximal flexural strength (148.36 MPa), and maximal density (2.66
g/cm3) at sintering temperature of
1360 oC. Whereas, the BaCO3 and MnCO3 co-substitution for the small amount of coal gangue
(prescription 9#) lead to an optimal effect. That is, minimal water absorption (0.02%),
maximal flexural strength (156.12
MPa) and density (2.68 g/cm3) are achieved at sintering temperature of 1360 oC. The optimal sintering
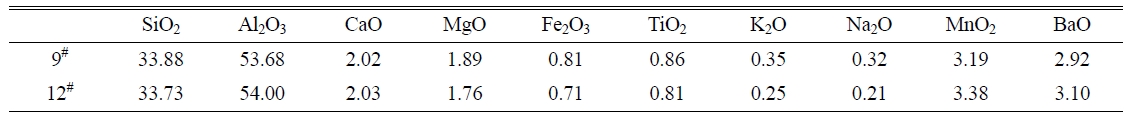
temperature for these samples is 1360 oC. The chemical compositions of the
prescription 9# and 12# are listed in Table 4.
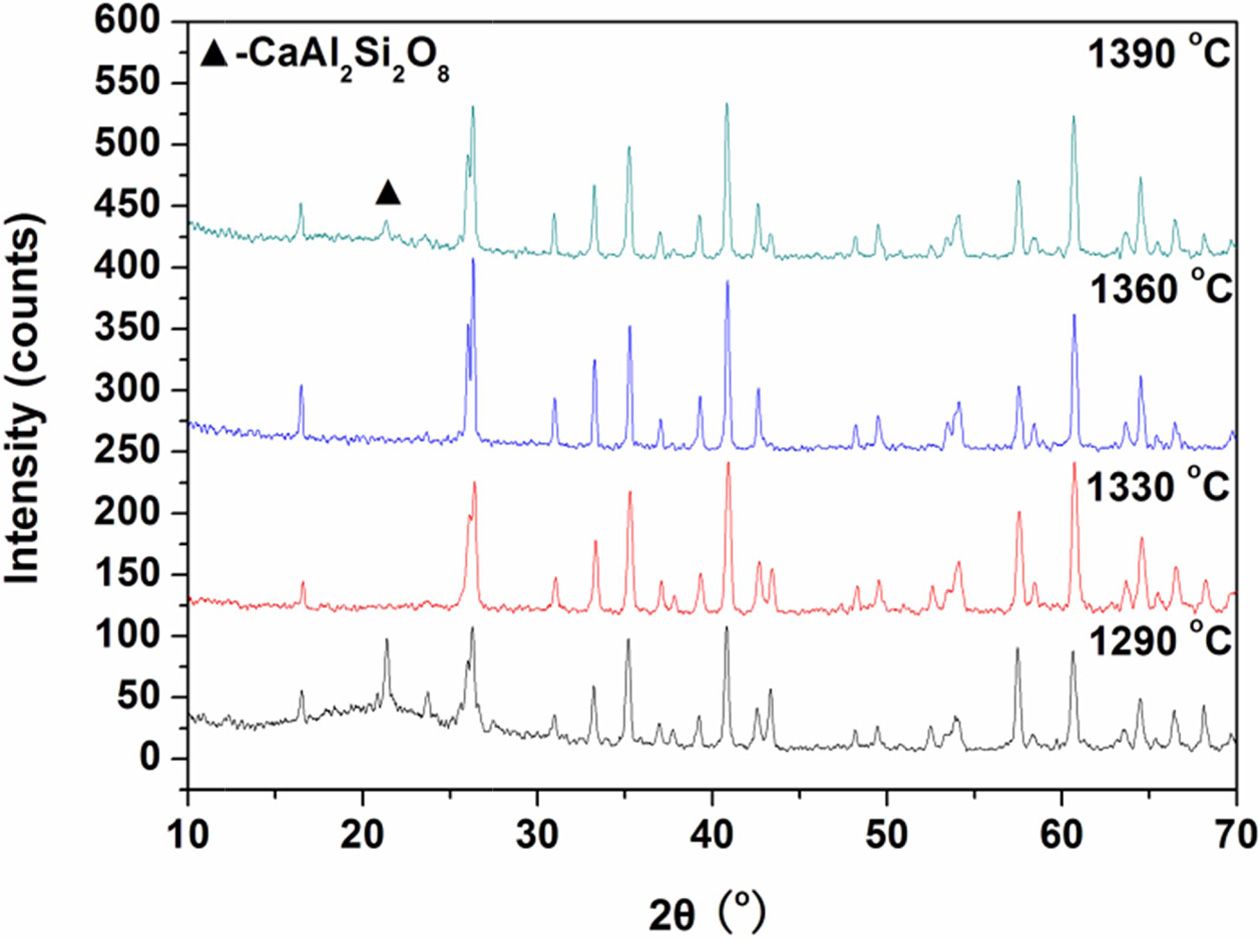
Fig. 5 shows the XRD patterns of the
samples fabricated with prescription 12#. The glass-ceramics sintered at 1330 oC and 1360 oC only contain mullite (Al6Si2O13) (JCPDS: 15-0776). When sintered at 1290 oC and 1390 oC, second phase svyatoslavite (CaAl2Si2O8) (JCPDS:
46-1266) appeared. The mullite is generally formed at the higher
temperature, such as above 1320 oC as reported in previous
literature [23]. The formation of mullite in the glass-ceramics at
the sintering temperature
of 1290 oC may be due to fine raw materials, fluxing actions of minerals
additives and mineralizers, and nucleation of Fe2O3 and TiO2 existing in the raw materials [24]. The mullite shows a compressive strength
(0.69 GPa) larger than many other crystals including the cordierite (0.32 GPa),
the boron nitrate (0.32 GPa), and the forsterite (0.55 GPa) [25]. This makes
the glass-ceramics samples high-strength. Thus, the glass-ceramics sintered at
1330 oC and 1360 oC show better properties.
Furthermore, the XRD peaks are enhanced as the increase in sintering
temperature from 1330 oC to 1360 oC, indicating the increase in the content of the mullite crystal. This
results in the optimal sintering temperature of 1360
oC.
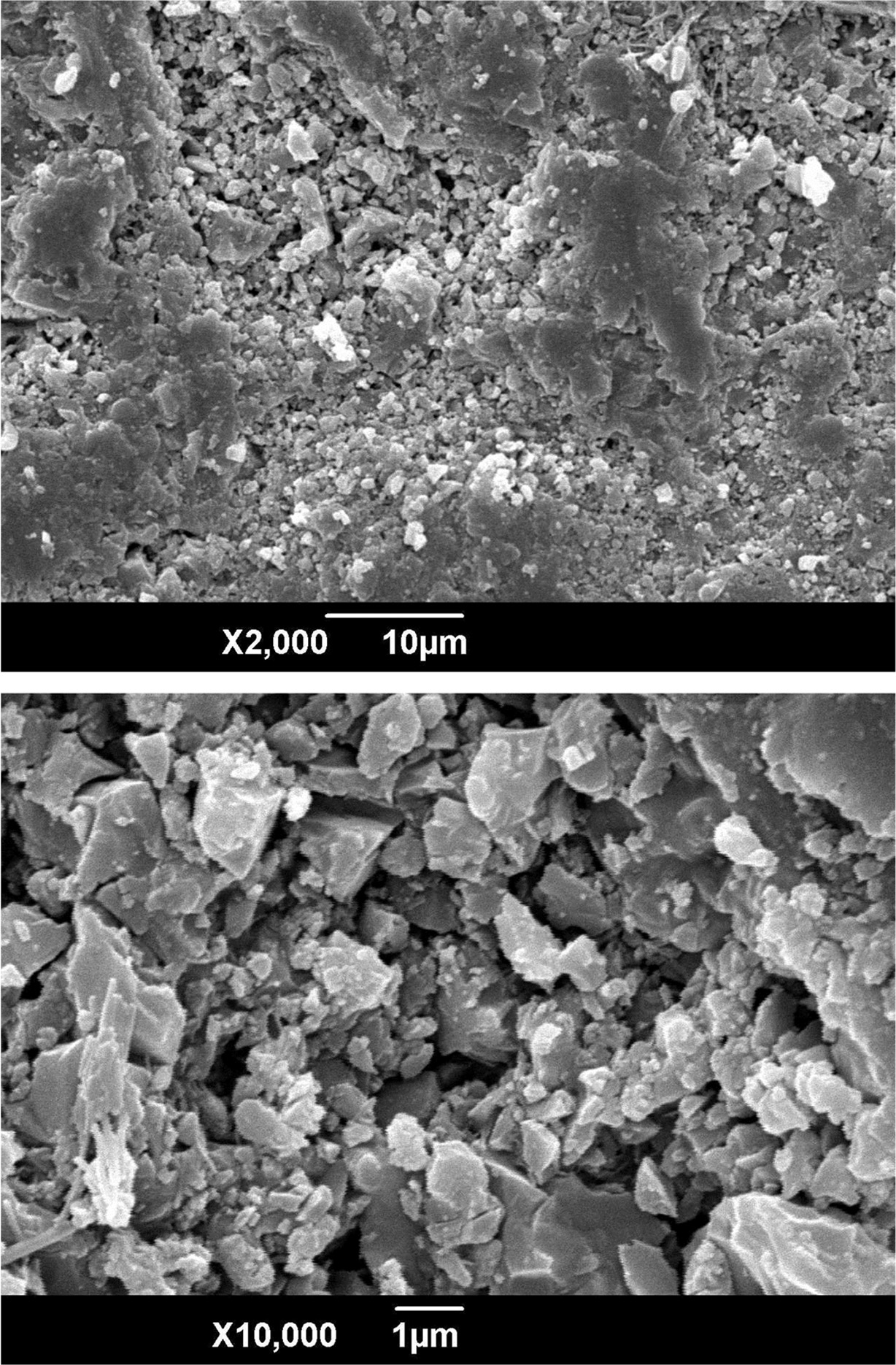
Fig. 6 shows the SEM micrographs of
the glass-ceramics fabricated with prescription 12# at sintering temperature of 1360 oC. The small and compact crystals with polygonal morphology can be
observed.
To optimize the processing parameter, the prescription 12# was further studied in various
processing parameters including milling time, forming pressure, and sintering time. The
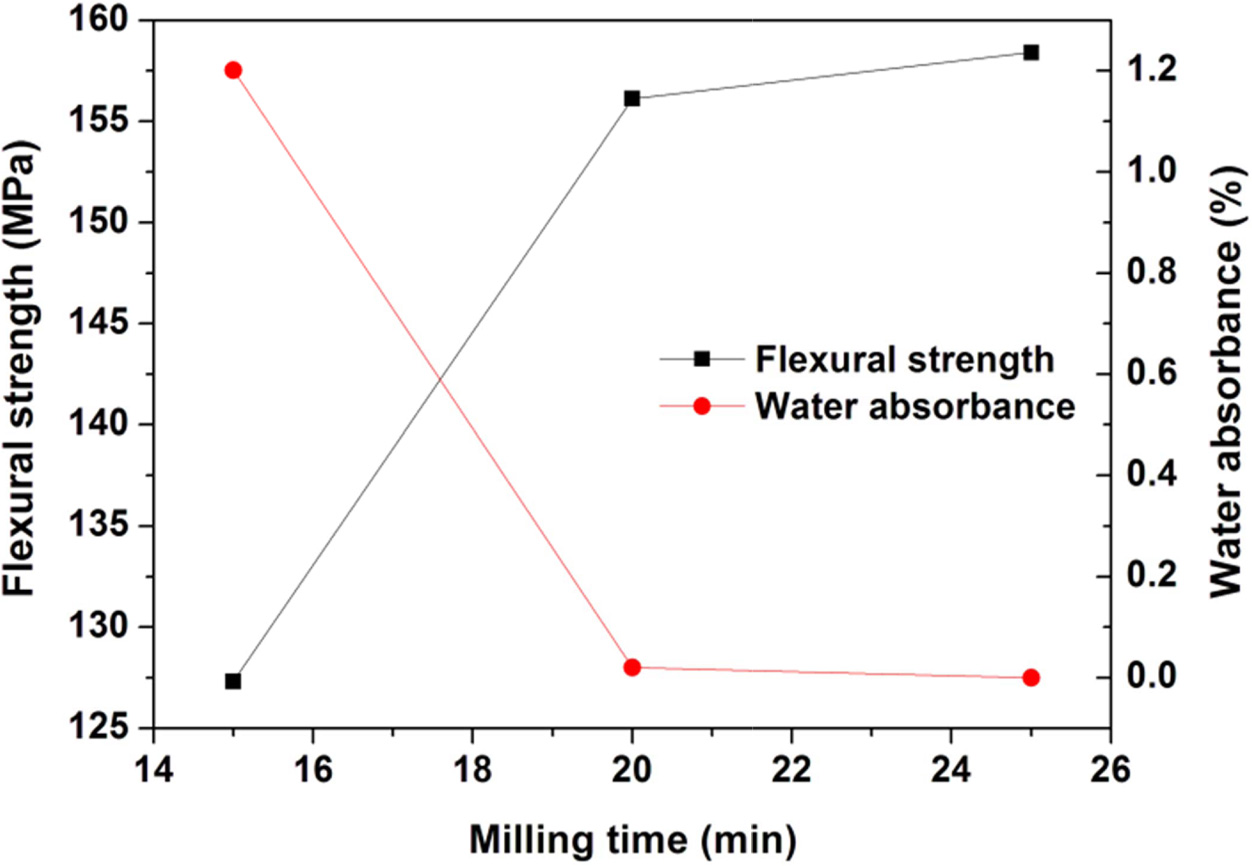
results are shown in Fig. 7, 8, and 9. The increase of milling time from 15 to
20 min leads to great enhancement of the properties,
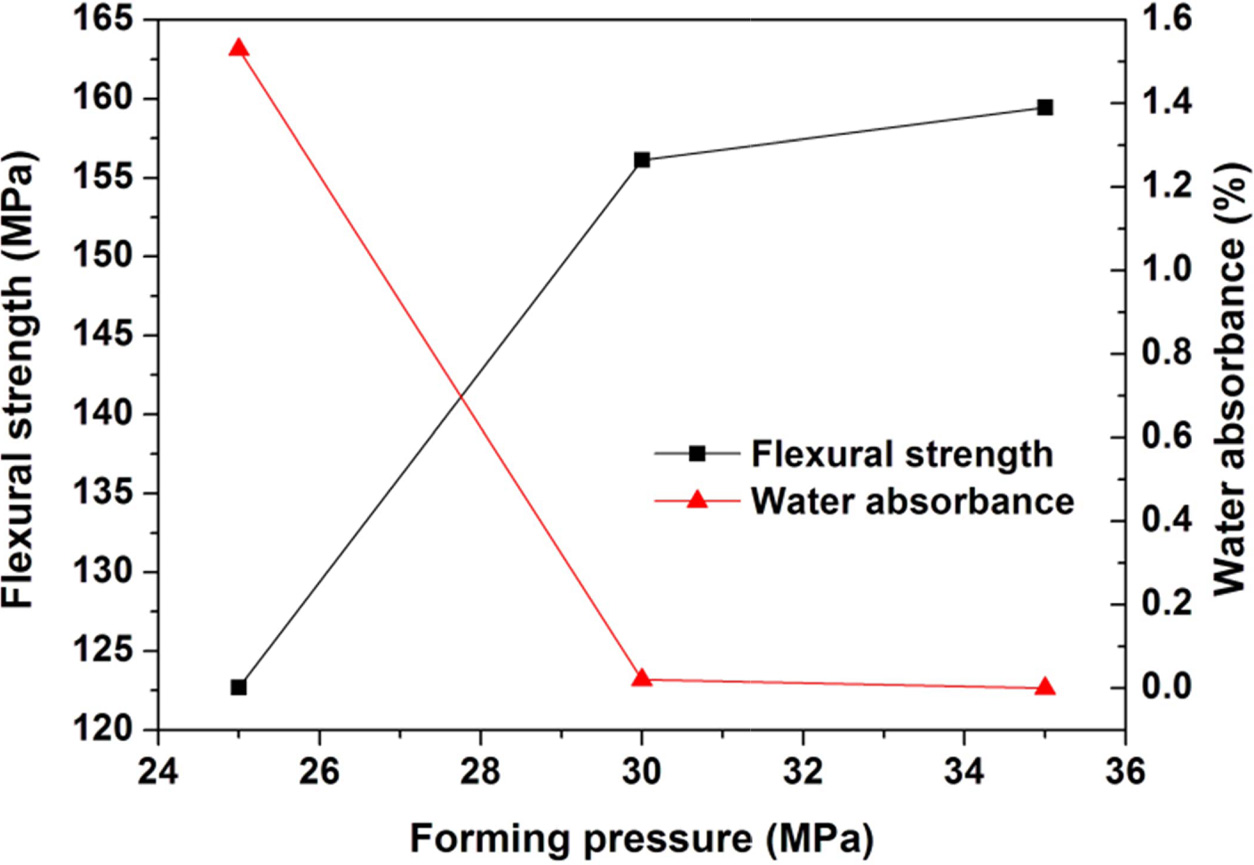
however further increase of milling time only shows a slight effect on the properties. Similarly, the
increase of forming pressure from 25 to 30 min leads to great enhancement of the properties
of the glass-ceramics, however further increase of forming
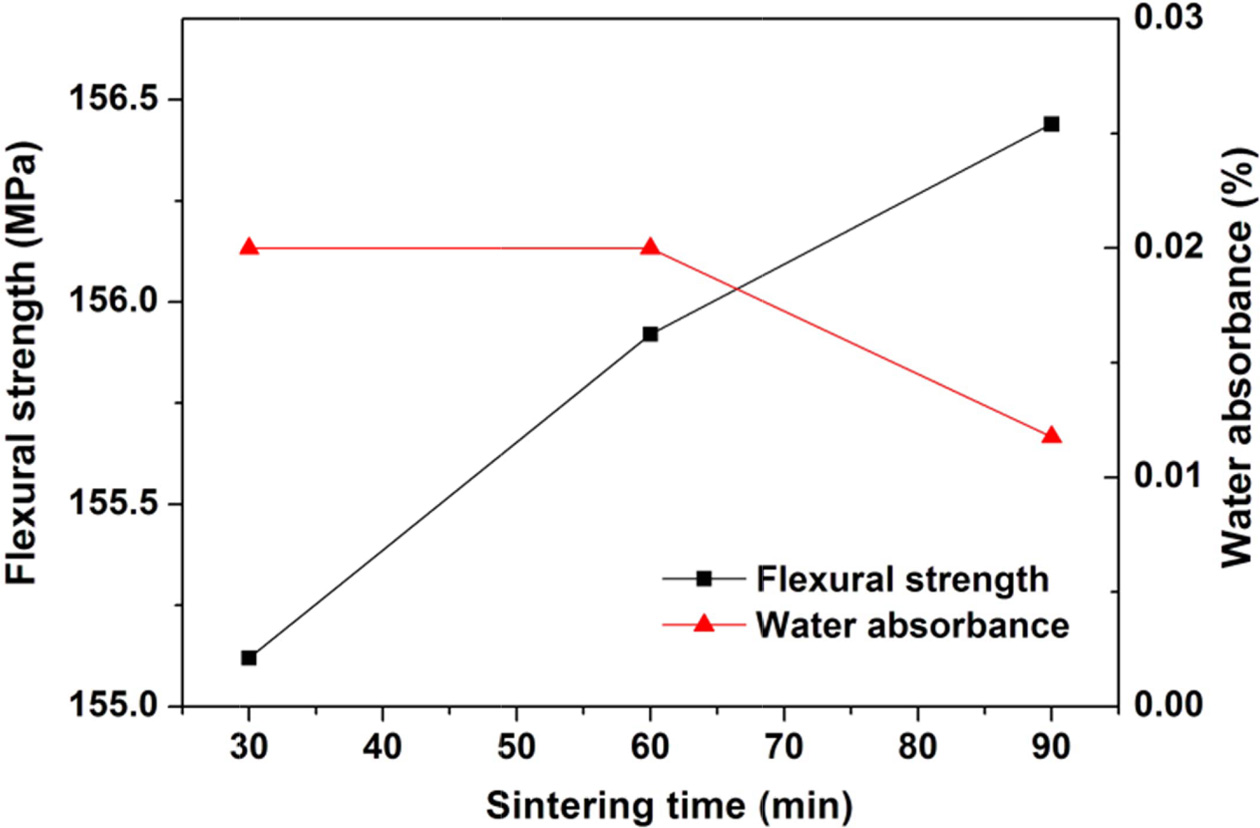
pressure only shows a slight effect. The increasing sintering time from 30 min
to 90 min only results in slight increase (~1.4 MPa) of flexural strength and a slight decrease
(0.01%) of water absorbance.
Many researchers have fabricated the glass-ceramics with various waste minerals [26-32]. Their glass-ceramics generally had the flexural
strength in the range of 30-130 MPa [26-32] and the density in the range of
~1.8-3.0 g/cm3 [26, 27, 30-32]. The glass-ceramics fabricated with coal gangue contained the
gehlenite as a main crystal phase and nepheline as the next crystal phase [18]. The glass-ceramics only
showed a bending strength of 28 MPa [18]. The mullite glass-ceramics fabricated with coal
gangue and γ-Al2O3 showed the bending strength from
64 MPa to 218 MPa as adding the La2O3 from
0 mol% to 10 mol% [16]. The porous mullite ceramic fabricated from coal gangue and bauxite showed the flexural
strength of 66.06 to 133.61 MPa varied with corn starch content [22]. The crystal phases different from mullite and their sizes and densities
are the key factors. By comparison, our glass-ceramics have higher strength. Fig. 3 Fig. 4 Fig. 8 Fig. 9
|
Fig. 1 Variations of (a) flexural strength and (b) water absorbance of the glass-ceramics fabricated with basic prescription 1-5# with sintering temperature (Sintering time: 30 min; milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 2 Variations of flexural strength of the glassceramics fabricated with prescription 6-12# with sintering temperature (Sintering time: 30 min; milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 3 Variations of water absorbance of the glassceramics fabricated with prescription 6-12# with sintering temperature (Sintering time: 30 min; milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 4 Variations of the density of the glass-ceramics fabricated with prescription 6-12# with sintering temperature (Sintering time: 30 min; milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 5 XRD patterns of the glass-ceramics fabricated with prescription 12# at different sintering temperatures for 30 min (Milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 6 SEM micrographs of the glass-ceramics fabricated with prescription 12# at sintering temperature of 1360 oC for 30 min (Milling time: 20 min; forming pressure: 30 MPa). |
|
Fig. 7 Variations of flexural strength and water absorbance of the glass-ceramics fabricated with prescription 12# at sintering temperature of 1360 oC for 30 min with wetmilling time (Forming pressure: 30 MPa). |
|
Fig. 8 Variations of flexural strength and water absorbance of the glass-ceramics fabricated with prescription 12# at sintering temperature of 1360 oC for 30 min with forming pressure (milling time: 20 min). |
|
Fig. 9 Variations of flexural strength and water absorbance of the glass-ceramics fabricated with prescription 12# at sintering temperature of 1360 oC with sintering time (Milling time: 20 min; forming pressure: 30 MPa). |
The waste coal gangue has been utilized for the fabrication of high-strength
glass-ceramics. Optimal utilization ratio of coal gangue was first
investigated. The effects of mineral additives, mineralizers,
processing parameters of forming and sintering of glass-ceramics were also researched. The optimal utilization ratio of the coal gangue
was determined to be 55 wt.% when the appropriate amounts of bauxite, clay, limestone, and steatite were used.
The appropriate amounts of mineralizers BaCO3, MnCO3,
MgCO3 can improve the
property of the glass-ceramics, in which BaCO3 and MnCO3
codoping and substitution show an optimal effect
as their amounts are 3 wt.%, respectively. The process optimization showed that
reasonable forming process parameters are milling time of 20 min and forming
pressure of 30 MPa, while optimal sintering temperature and time are 1360
oC and 30 min, respectively. Further increases of the milling time,
forming pressure and sintering time only lead to slight enhancements of the
properties. In the condition of these reasonable process parameters, the sintered glass-ceramics were of high strength (156.12 MPa), low density (2.68 g cm-3), and low water absorption (0.02
%). This makes the sintered glass-ceramics an excellent glass-ceramics having
potential superiority for the applications
in proppant materials, building materials, cooking ceramics, et al..
This work supported by Scientific Research Program Funded
by Shaanxi Provincial Education Department (Program No.19JK0431)
- 1. J. Temuujin, U. Bayarzul, E. Surenjav, K.D. Sung, and C.Y. Sik, J. Ceram. Proc. Res. 18[2] (2017) 112-115.
- 2. K.D.T. Kien, P. DinhTuan, T. Okabe, D.Q. Minh, and T.V. Khai, J. Ceram. Proc. Res. 19[6] (2018) 472-478.
- 3. I. Krstić, S. Zec, and V. Lazarević, Sci. Sinter. 50 (2018) 139-147.
-

- 4. O.R.K. Montedo AI.T. lves, and C.A. Faller, Mater Res Bull. 72 (2015) 90-97.
-

- 5. A. Mirza, M. Riaz, and T. Hussain, J. Alloys Compds. 726 (2017) 348-351.
-

- 6. V. Karayannis, A. Moutsatsou, and A. Domopoulou, J. Build Eng. 14 (2017) 1-6.
-

- 7. J.-B. Lee, S.-S. Kim, J.-Y. Lee, and J.-S. Ryou, J. Ceram. Proc. Res. 18[4] (2017) 291-300.
- 8. H.S. Lee and K.H. Sho, J. Ceram. Proc. Res. 19[2] (2018) 105-110.
- 9. M. Amiri Roudan, S. Ramesh, A. Niakan, Y.H. Wong, M. Akhtari Zavareh, Hari Chandran, W.D. Teng, N. Lwin, and U. Sutharsini, J. Ceram. Proc. Res. 18[1] (2017) 69-72.
- 10. T. Terzić, N. Đorđević, M. Mitrić, S. Marković, K. Đorđević, and V.B. Pavlović, Sci Sinter. 49 (2017) 23-37.
-

- 11. G. Patridge, Glass Technol. 35[3] (1994) 116-126.
-

- 12. X.Y. Cong, S. Lu, Y. Yao, and Z. Wang, Mater. Des. 97 (2016) 155-162.
-

- 13. C.-l. Wang, W. Ni, S-Q. Zhang, S. Wang, G.-S. Gai, and W.-K. Wang, Constr. Build. Mater. 104 (2016) 109-115.
-

- 14. D.-X. Li, X.-Y. Song, C.-C. Gong, and Z.-H. Pan, Cem. Concr. Res. 36[9] (2006) 1752-1759.
-

- 15. M.-Z. Liu, Z.-W. Zhu, Z. Zhang, Y.-C. Chu, B. Yuan, and Z.-L. Wei, Sep. Purif. Technol. 237 (2020) 116483.
-

- 16. H.-P. Ji, M.-H. Fang, Z.-H. Huang, K. Chen, Y.-G. Xu, Y.-G. Liu, and J.-T. Huang, Ceram. Int. 39[6] (2013) 6841-6846.
-

- 17. W. Pannhorst, J. Non-Cryst. Solids 219 (1997) 198-204.
-

- 18. M. Yang, Z.-X. Guo, Y.-S. Deng, X.-L. Xing, K.-H. Qiu, J.-P. Long, and J.-F. Li, Inter. J. Miner. Process. 102-103[25] (2012) 112-115.
-

- 19. L.-Q. Luo, K.-Y. Li, W. Fu, C. Liu, and S.-Y. Yang, Constr. Build. Mater. 232 (2020) 117250.
-

- 20. W. Ni, R-Y. Gong, and C.-W. Li, Fuel and Energy Abstracts 39[4] (1998) 304.
-

- 21. F. Fang, Fuel and Energy Abstracts 39[3] (1998) 193.
-

- 22. J.G. Rose, Resources and Conservation 9 (1982) 119-129.
-

- 23. Y. Manama, S. Aoki, N. Camshare, and K. Nina, J. Am. Ceram. Soc. 78[5] (1995) 1265-1271.
-

- 24. H.-Y. He, J. Wuhan Univ. Technol.-Mater. Sci. Ed. 14 (1999) 28-34.
- 25. A.H. Jones and R.A. Cutler, Terra Tek, Inc, US, 1980.
- 26. E. Bernardo, L. Esposito, E. Rambaldi, A. Tucci, Y. Pontikes, and G.N. Angelopoulos, J. Europ. Ceram. Soc. 29 (2009) 2921-2927.
-

- 27. T. Toya, Y. Tamura, Y. Kameshima, and K. Okada, Ceram. Int. 30 (2004) 983-989.
-

- 28. H.-Y. Liu, H.-X. Lu, D.L. Chen, H.L, Wang, H.L. Xu, and R. Zhang, Ceram. Int. 35 (2009) 3181-3184.
-

- 29. E. Bernardo, R. Castellan, and S. Hreglich, Ceram. Int. 33 (2007) 27-33.
-

- 30. Y.J. Park and J. Heo, J. Hazard. Mater. B91 (2002) 83-93.
-

- 31. D.-L. Shu, Mechanical Property of Engineering Materials, China Machine Press, Beijing, 2004, pp. 214-228.
-

- 32. W.R. Beck and R.B. Castle, Proppant for well fractures and method of making same, US 4493875, 1985, Jan. 15.
- 33. Q.-K. Lü, X-F. Dong, and Z.-Y. Zhu, J Hazard Mater. 273 (2014) 136-145.
-

 This Article
This Article
-
2020; 21(1): 69-74
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.69
- Received on Aug 21, 2019
- Revised on Jan 23, 2020
- Accepted on Feb 5, 2020
 Services
Services
- Abstract
introduction
experimental procedures
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- H.-Y. He
-
College of Material Science and Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China
Tel: +86-15319453608 - E-mail: hehy@sust.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.