- Improved performance of barium cobalt-based perovskites materials: Influence of B-site substitution and metal oxide supported perovskite on oxygen desorption property and reactivity
Shian Lia, Rongqiang Wei, Yuhang Jiang, Qiuwan Shena,*, Guogang Yanga,* and Naibao Huangb
aMarine Engineering College, Dalian Maritime University, Dalian, China
bCollege of Transportation Engineering, Dalian Maritime University, Dalian, China
Oxy-fuel combustion is one of
the proposed technologies which have the potential to achieve a zero CO2
emission. To enhance the oxygen production performance of the oxygen carrier,
different LaBO3-δ (B=Co, Ni, Fe, Cr) and metal oxide (CeO2,
Al2O3, ZrO2) supported BaCoO3-δ perovskites
have been successfully synthesized by the EDTA sol-gel method and further
applied for producing oxygen. The oxygen desorption/production performance of
synthesized perovskites were studied in a fixed-bed reactor system.
Furthermore, the effects of H2O and air as regeneration gas of metal
oxide supported BaCoO3-δ perovskite oxygen carrier were investigated
in detail. Results shows that the oxygen desorption amount of different B-site
substituted LaBO3-δ (B=Co, Ni, Fe, Cr) perovskites decrease in the order
of LaNiO3-δ > LaCoO3-δ > LaCrO3-δ >
LaFeO3-δ.While compared with pure BaCoO3-δ and different
metal oxide supported BaCoO3-δ, CeO2 supported BaCoO3-δ
features higher production amount of oxygen. Multiple cycles demonstrated that
BaCoO3/CeO2 displays higher stability and regeneration
capacity, which is the key factor to provide stable O2/CO2
gas stream for oxyfuel combustion application. In short, the novel BaCoO3/CeO2
oxygen carrier developed in this work exhibits high oxygen desorption
capacity and stability. In addition, it provides a promising potential for
oxygen production in industrial application.
Keywords: CO2 capture; Oxygen carrier; Supported-perovskite; Oxygen production
The combustion of fossil fuels contributes to the emission
of carbon dioxide into the atmosphere, leading to
global warming [1, 2]. Oxyfuel combustion technology is
a very effective technology path to reduce carbon dioxide emissions [3-5]. This
type of combustion requires a high concentration of oxygen. The current
cryogenic process is the only commercially available way to provide large-scale pure
oxygen, but the large investment in oxygen production and high
energy consumption is its major economic challenge and drawback. For oxyfuel
combustion, only an O2/CO2 mixture gas with an oxygen
concentration of 30% ~40% is required for the fuel combustion but not the pure
oxygen.
Perovskite-like oxides-ABO3 (where A and B are usually
rare earth and transition metal cations, respectively) can be
tailored to create a wide family of catalysts by varying either the A-site or
the B-site metal ion. Perovskite-type oxides have been investigated intensively
as functional materials because of their potential technology in
the applications such as fuel cells, a component of
capacitors, microwave technology, electrodes, and immobilization of
nuclear wastes, as well as being catalysts for oxidation
and hydrogenation [6-12].
A new application of producing pure O2 or O2/CO2
gas streams by using perovskite-type oxygen carriers for oxyfuel
combustion is proposed by Lin et al. [13]. The reversible
adsorption/desorption processes based on the perovskite-type oxygen carrier is
described as below: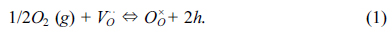
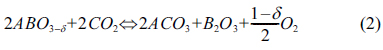
Where VO··
is the oxygen vacancy, OO×
and □· denote
lattice oxygen and electronic-hole, respectively.
A relatively low oxygen desorption amount may be a major
drawback of this technique. This problem may pose a challenge to the high
efficiency of the oxygen product volume in practical applications. Therefore,
the development of perovskite-type oxygen carrier materials
with excellent oxygen desorption performance and cyclic performance is
necessary.
A
common approach to improve the properties of perovskite-type
oxygen carrier is using A/B site substitution in the metal oxides. Our previous study demonstrated that barium cobalt-based (Ba-Co-O) perovskites
oxygen carriers present higher oxygen
desorption capability and oxygen
releasing rate [3, 4]. As La-based perovskites are the most common perovskites, LaBO3-δ (B=Co, Ni, Fe, Cr)
and BaCoO3-δ are selected as candidate oxygen carrier in this study
[14, 15]. CeO2-based materials are of intensive interest because of their outstanding
oxygen storage capacity, and CeO2
can supply active oxygen species to
perovskite [16, 17]. To support BaCoO3, zirconia (ZrO2) is particularly adopted because ZrO2
remains highly stable under oxidizing
and reducing atmospheres, making ZrO2
as a promising support [18, 19].
Moreover, Al2O3 is another typical support, and the use of a load material having high thermal
conductivity can reduce the sintering of the oxygen carriers. The additives can improve reaction performance and
stability for perovskites materials
in the oxygen-permeable membrane application [20, 21]. However, there is
rare research about using supports to improve the reaction performance of
perovskites. The effect of the supports on the reaction and the oxygen
desorption capability are still unclear, and the durability of the supported
perovskites has not yet been developed.
The current work aimed to
study the effects of B-site (B=Co, Ni, Fe, Cr) substitution on oxygen
desorption performance for La-based perovskites and the effect of supports on
oxygen production/cyclic performance of metal oxide supported BaCoO3-δ.
All the perovskite-type oxides were synthesized
by EDTA sol-gel combustion method. The reactivity and performance of different
pure and supported perovskites were investigated in a fixed-bed reactor system.
Oxygen
carrier preparation
The pure LaBO3-δ (B=Co, Ni, Fe, Cr) and BaCoO3-δ
perovskite samples were synthesized by EDTA sol-gel method as shown in our
previous study [5]. And metal oxide supported perovskite oxygen carriers in
this study were prepared also by a sol-gel synthesis method. The detail
preparation process for BaCoO3-δ /ZrO2 for example is as
follows: Metal nitrates Ba(NO3)2 and Co(NO3)2 · 6H2O
were used as the raw materials and all of analytical purities. A design amount
of metal nitrates and citric acid were dissolved in the NH3-EDTA
solution. The mole ratios of EDTA: citric acid: total metal ions
were controlled as 1:1.5:1. A desired amount of ZrO2 was added in
the string precursor solution. The solution was then gently heated and stirred
at 70 oC for 5 h and further dried at 105 oC
for 10 h, respectively. Then the dry residual was calcined at 850 oC
for 8 h. Finally, the resultant black powders were characterized and tested.
Experimental
procedure
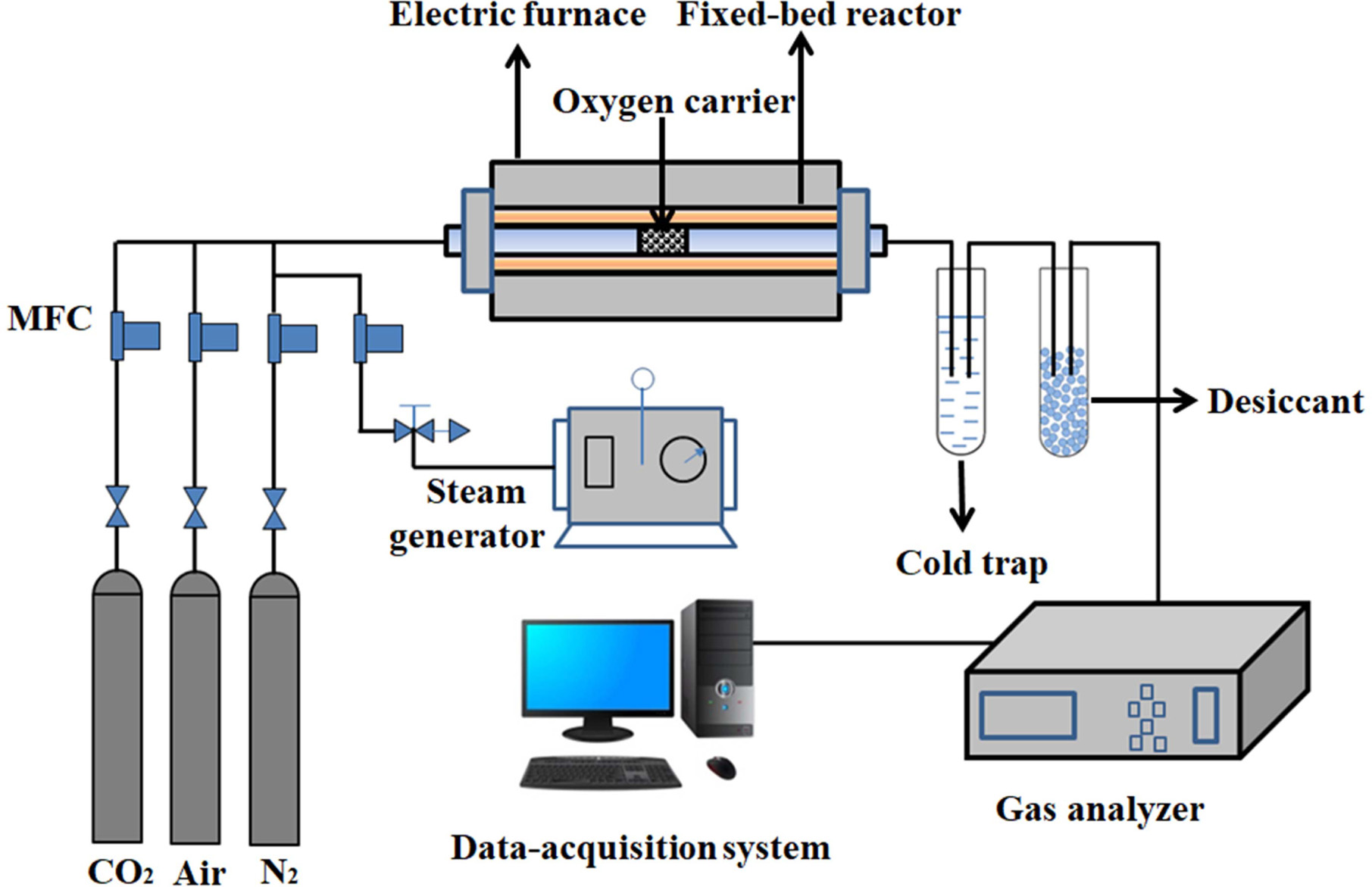
Oxygen adsorption/desorption experiments were performed in
a fixed-bed reactor system as shown in Fig. 1. It consisted of a gas feeding
system (including N2, air, CO2 and a steam
generator), an electric furnace with a quartz reactor, a gas analyzer and a
computerized data-acquisition system.
About 5.0 g of synthesized perovskite powder is filled in
the middle of the quartz reactor. Air/H2O and CO2 are
used as the feed gas for the adsorption step and the purge gas for the
desorption step, respectively.
Output
calculation
The total oxygen desorption amount calculation is
performed by an integration scheme based on the obtained oxygen concentration
distribution. And the following formula can be used:

where ΣCO2 is the integration of the entire oxygen
concentration during the desorption, Fout (L/s) is the flow
rate of desorption effluent, m (g) is the mass of perovskite powders, VO2 (mL/g · sample)is
the oxygen desorption amount for 1 g of the perovskite sample.
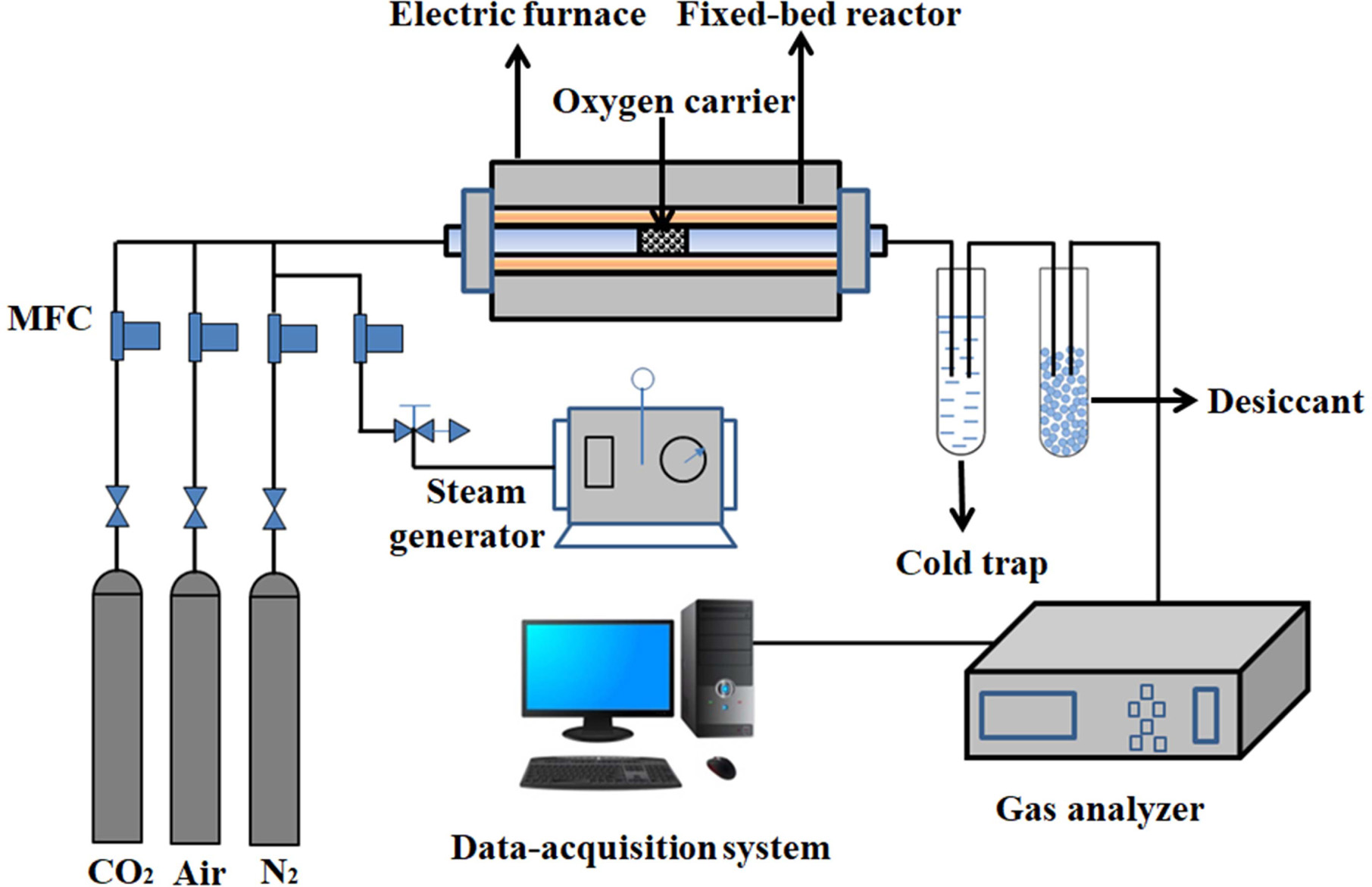
|
Fig. 1 Schematic diagram of fixed bed reactor system. |
Effects
of B-site substitution on oxygen desorption performance for la-based
perovskites
The effects of different B-site substitution by different transition
metal ions on the oxygen production performance of LaBO3-δ
were investigated. Fig. 2 demonstrates the comparison of the oxygen desorption
performance for LaBO3-δ (B = Co, Ni, Fe, Cr). It is obvious that
B-site total substitution has significant effect on the oxygen desorption performance
of LaBO3-δ. It shows that the oxygen desorption amount of B-site
total substitution for LaBO3-δ is in the following order:
LaNiO3-δ > LaCoO3-δ > LaCrO3-δ
> LaFeO3-δ. It indicates that LaNiO3-δ has
the optimum oxygen desorption performance among the above
B-site-substituted LaBO3-δ perovskite. The oxygen desorption amount
for LaNiO3-δ is about 13 ml O2/g×perovskite.
Effect
of metal oxide supported perovskite oxygen carriers on oxygen desorption
performance
The performance of metal oxide supported BaCoO3−δ
oxygen carriers was studied in the fixed-bed reaction. The absorption and
desorption temperature are 800 oC and 850 oC
respectively.
Its performance was compared with the pure BaCoO3−δ
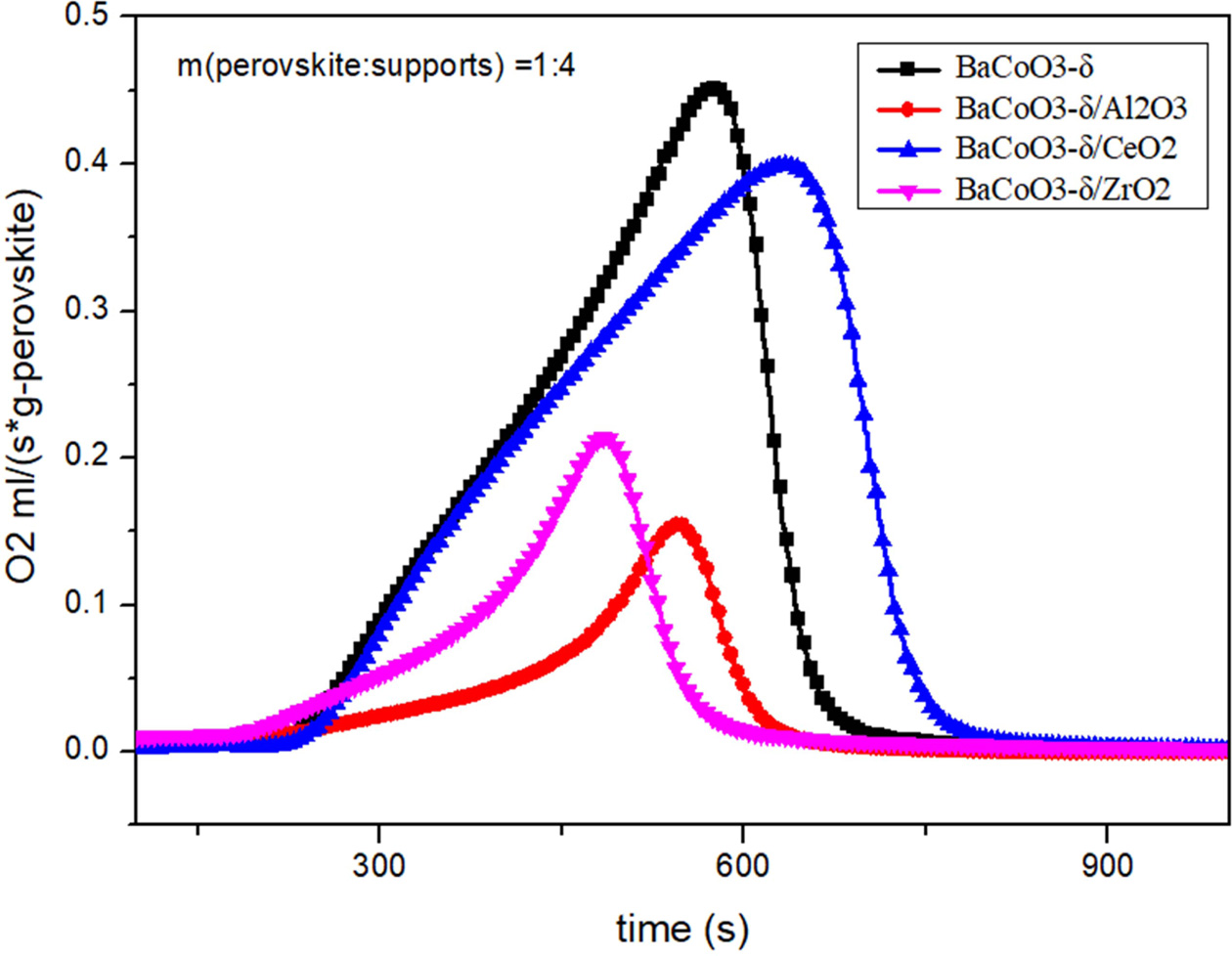
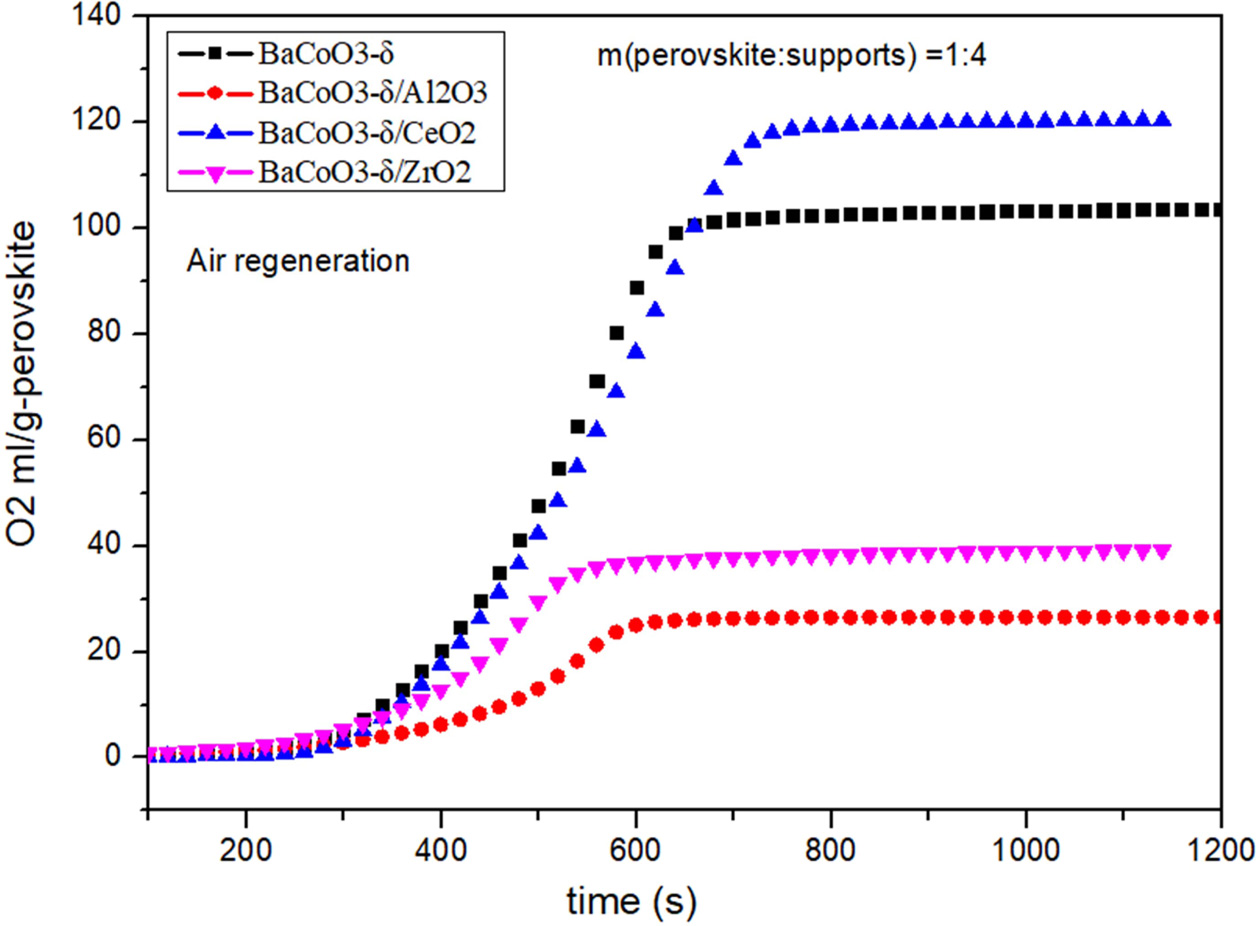
oxygen carriers. Comparison of oxygen desorption curves
and oxygen desorption amount of pure BaCoO3−δ, BaCoO3−δ/CeO2
(BCC), BaCoO3−δ/Al2O3 (BCA) and BaCoO3−δ/ZrO2
(BCZ) are shown in Fig. 3 and Fig. 4. Results showed that the order of oxygen
production amount was as follows:
BCC > BC > BCZ > BCA. That
is to say, BCC perovskite has the improved oxygen desorption performance. On
the other hand, the Al2O3 and ZrO2 reduced the
oxygen desorption property of BaCoO3−δ oxygen carrier.
The results showed that CeO2-supported
perovskite has better oxygen production performance in the desorption process.
As an oxygen storage material, CeO2 exhibits high oxygen mobility
and oxygen capacity at high temperatures. The lattice oxygen in CeO2
can be supplied to perovskites in the high temperature process [22-24].
Therefore, CeO2 is an excellent support material for
oxygen carriers, which can enhance
the performance of BaCoO3−δ nanoparticles.
Effects
of H2O regeneration gas on oxygen desorption performance and cyclic
performance
Main feature of this type of perovskites is that they can
adsorb O2 from air at high temperature to restore its perovskite
structure. In order to study the self-recovery ability of perovskite carriers,
water vapor and air are chosen to compare their recycling regeneration capacity.
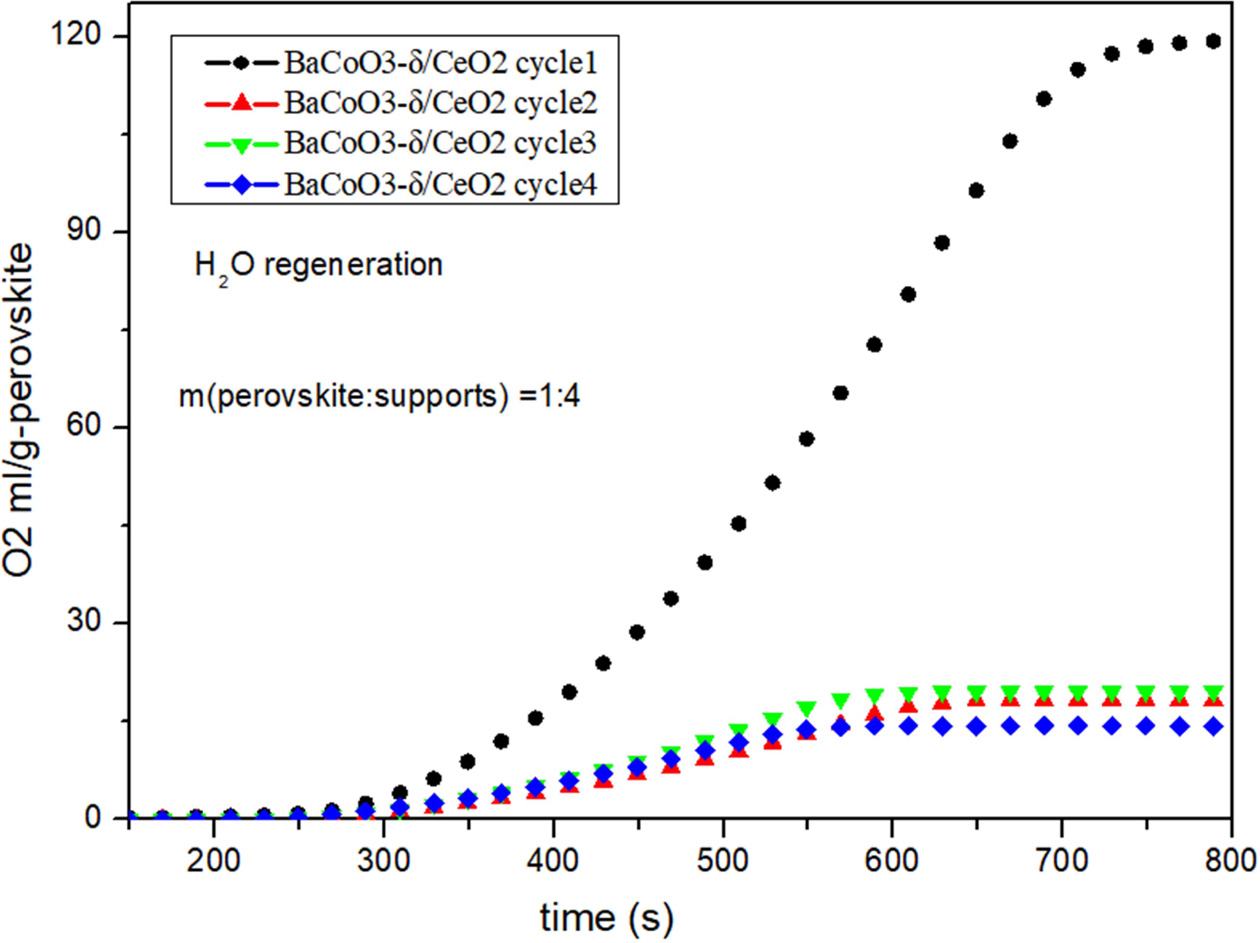
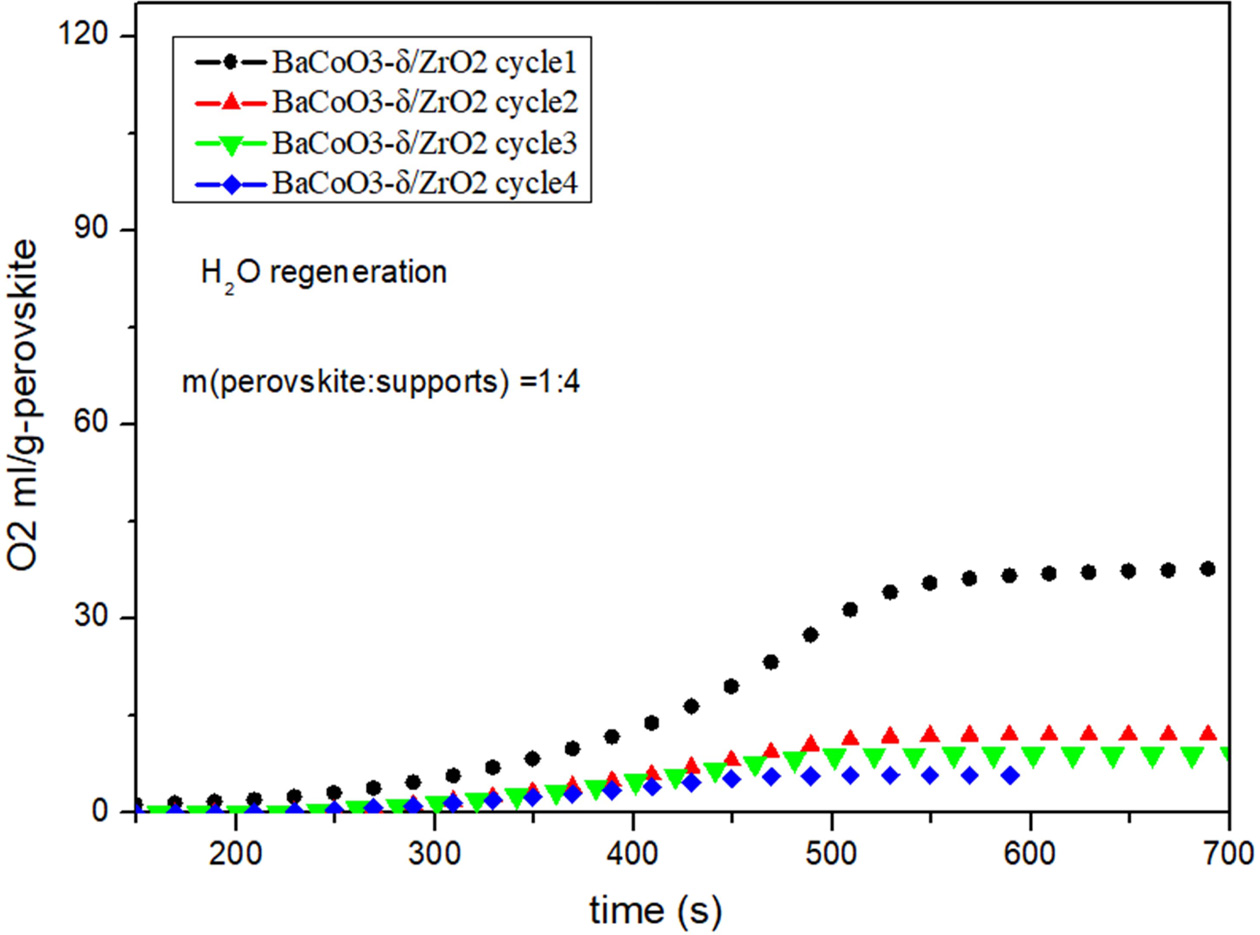
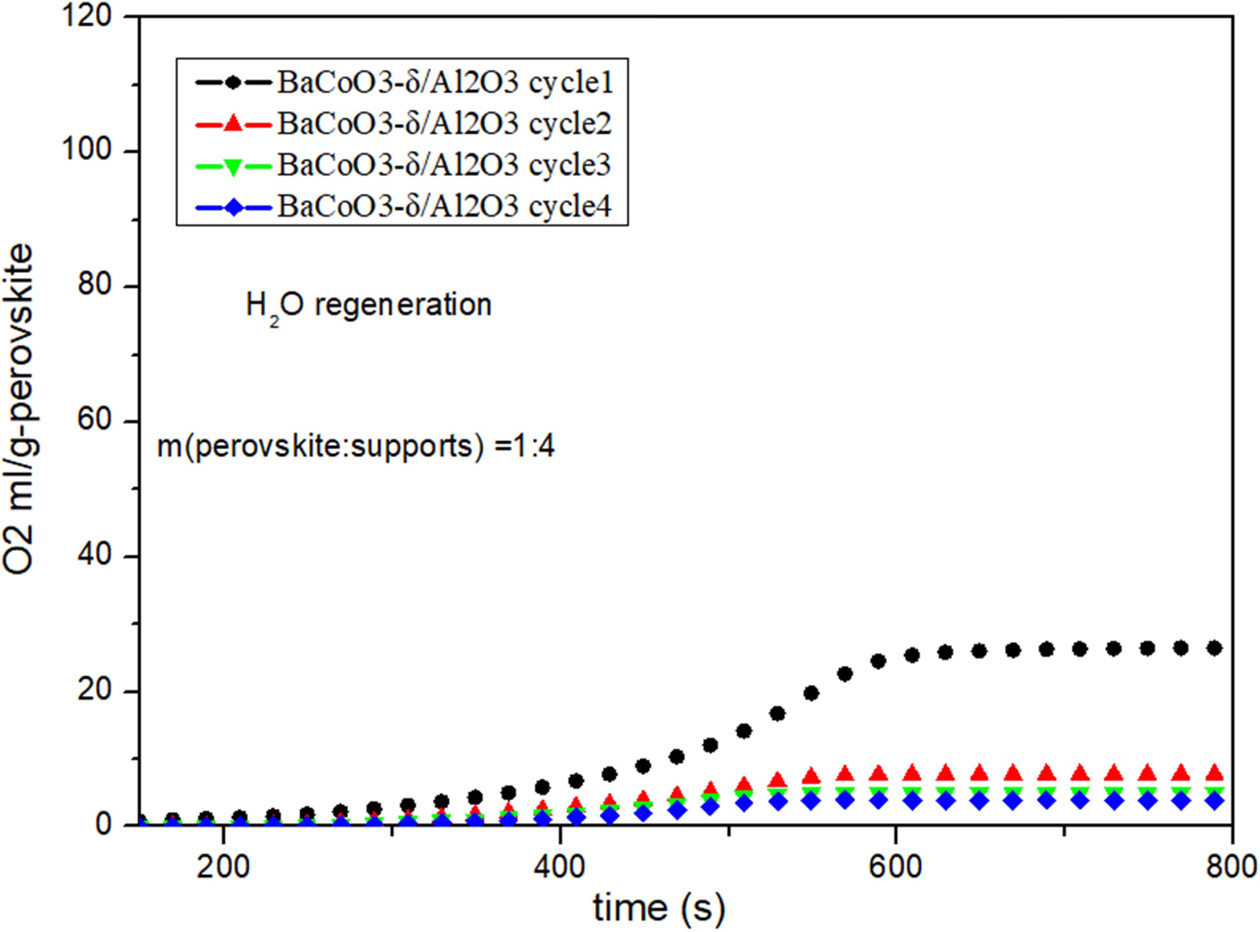
Fig. 5, Fig. 6, Fig. 7 and Fig. 8 show the cyclic
performance of pure BaCoO3−δ and supported BaCoO3−δ
oxygen carrier under the H2O instead of Air as regeneration gas
conditions. Results show that all the perovskite oxygen carriers
have good oxygen desorption performance in
the first cycle. However there is a significant drop during cycle 2 to cycle 4
for all the samples. It is indicated that H2O as
regeneration adsorbent does not appropriate for perovskite
recovery.
Cyclic
performance
Stability is a key parameter
for the performance of oxygen carriers, providing a stable O2/CO2
recycle gas for oxyfuel combustion
applications. This work evaluated the stability of the proposed BaCoO3-δ/CeO2
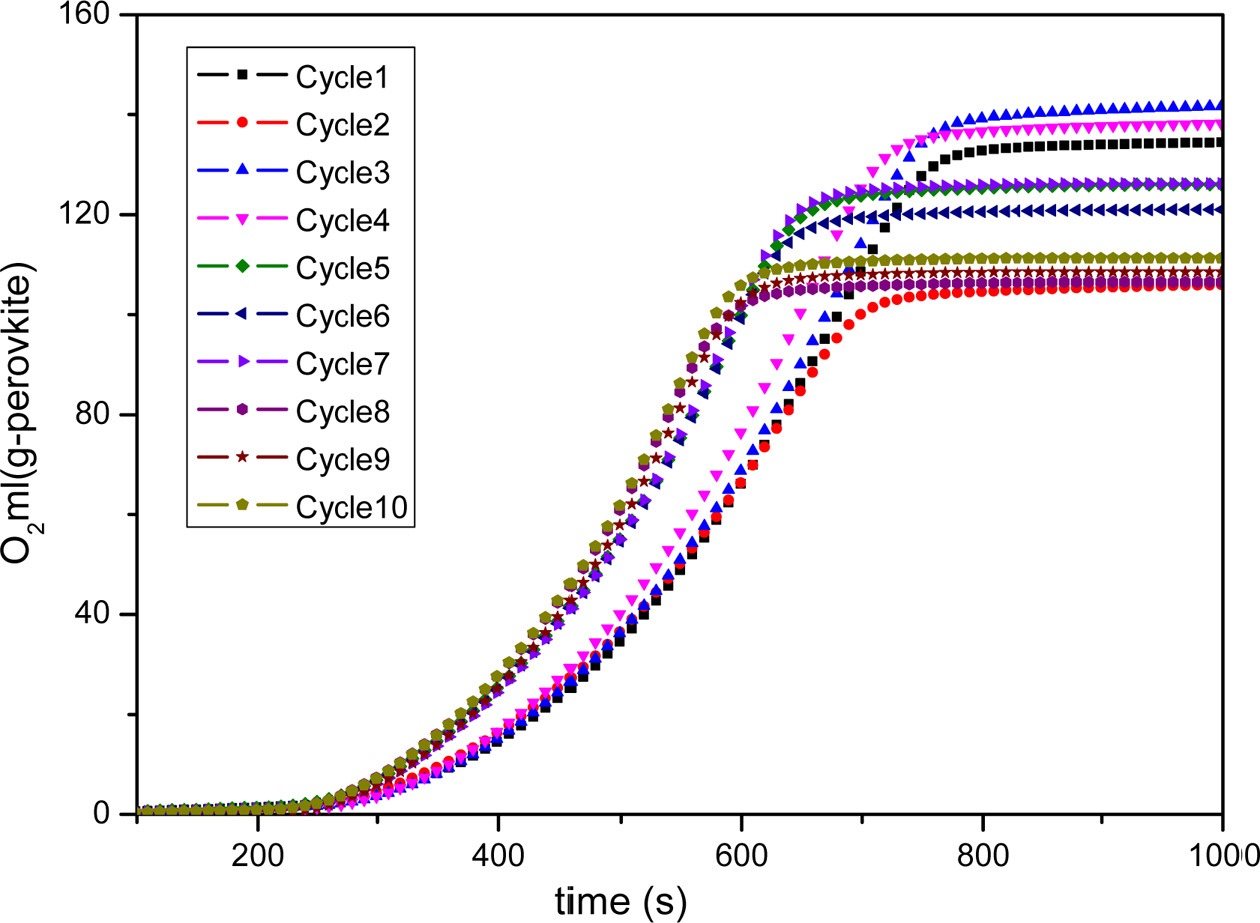
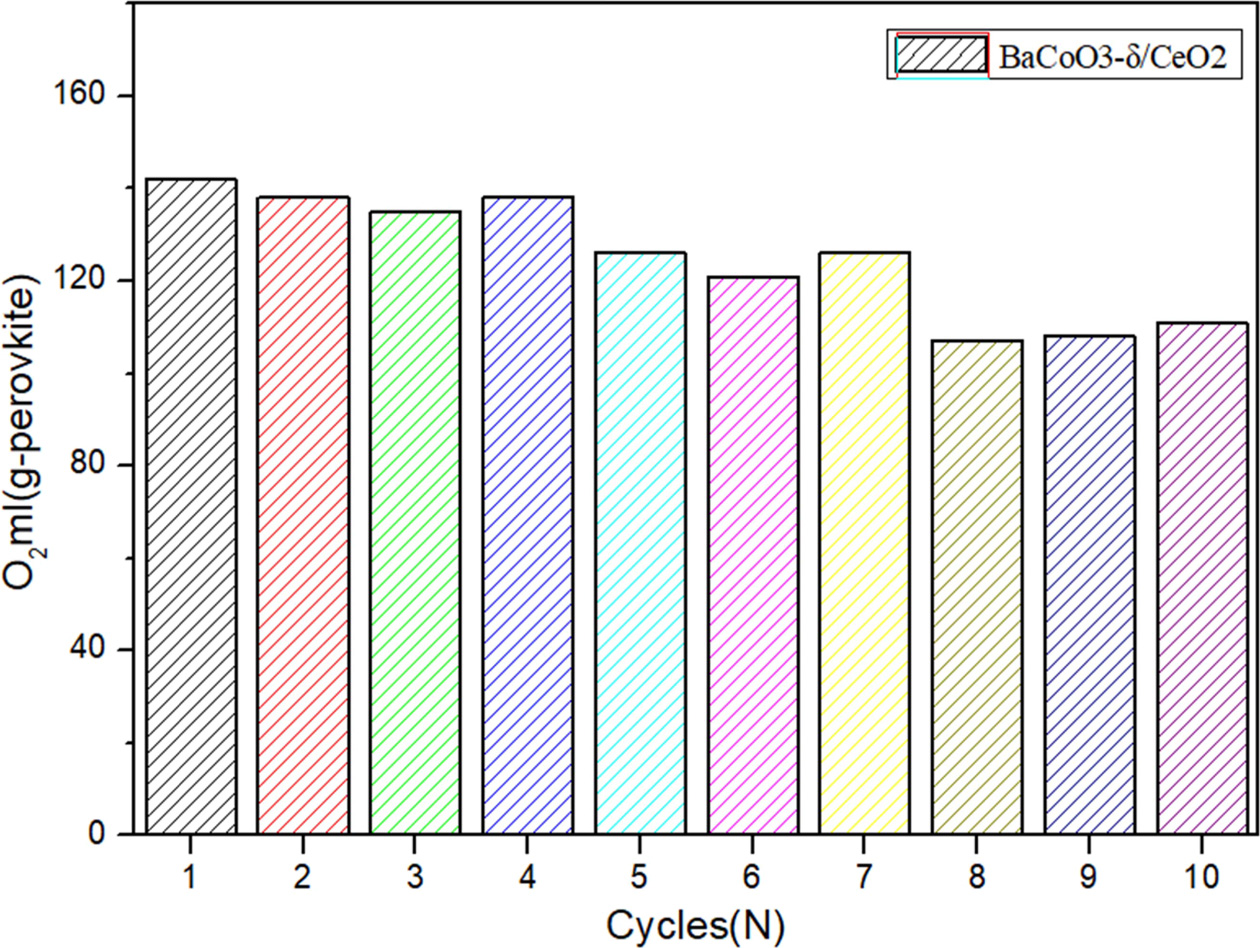
perovskite oxygen carrier and applied it for 10-cycles long-term testing. Fig.
9 and Fig. 10 shows the cyclic capacity of BaCoO3−δ/CeO2 perovskite
oxygen carrier. It indicates that the oxygen desorption performance does not
show an obvious decrease during the
1-7 cycles. After cycle 7, there is a litter decrease of the oxygen desorption
amount for BaCoO3−δ/CeO2.
The oxygen desorption amount of BaCoO3−δ/CeO2 could still
achieve 110 ml/g sample after 10 cycles. Therefore, the novel perovskite-type oxygen carrier BaCoO3−δ/CeO2 has
excellent regeneration capacity in cyclic use, which is very important for practical
application.
|
Fig. 2 Oxygen desorption curves for LaBO3-δ (B=Sr, Ba, Mg, Ca). |
|
Fig. 3 Comparison of oxygen desorption curves for pure and supported BaCoO3−δ. |
|
Fig. 4 Comparison of oxygen desorption amount of pure and supported BaCoO3−δ. |
|
Fig. 5 Cyclic performance of pure BaCoO3−δ (H2O as regeneration adsorbent). |
|
Fig. 6 Cyclic performance of BaCoO3−δ/CeO2 (H2O as regeneration adsorbent). |
|
Fig. 7 Cyclic performance of BaCoO3−δ/ZrO2 (H2O as regeneration adsorbent). |
|
Fig. 8 Cyclic performance of BaCoO3−δ/Al2O3 (H2O as regeneration adsorbent). |
|
Fig. 9 Long-term testing of BaCoO3−δ/CeO2 oxygen carrier. |
|
Fig. 10 Comparison of cyclic oxygen desorption amount of BaCoO3−δ/CeO2 oxygen carrier. |
In this study, different LaBO3-δ
(B=Co, Ni, Fe, Cr) and metal oxide (CeO2, Al2O3,
ZrO2) supported BaCoO3-δ perovskites have been successfully prepared by the EDTA sol-gel method and
applied for oxygen production. Results show that B-site total substitution has
significant effect on oxygen desorption
properties of LaBO3-δ. LaNiO3-δ has the optimum oxygen desorption
performance among the B-site-substituted LaBO3-δ
perovskite. Effects of H2O regeneration gas on oxygen
desorption performance and cyclic performance indicated that H2O
as regeneration adsorbent comparing to air
does not appropriate for perovskite recovery.CeO2 supported
perovskite featured better oxygen production
performance. Furthermore, BaCoO3−δ/CeO2 exhibits an
excellent regeneration capacity in cyclic use which is very important for practical
application.
The authors gratefully acknowledge the financial supports from National Natural Science Foundation of China (No.51606013 and No.51779025). This work is also funded by the Fundamental Research Funds for the Central Universities of China (No.3132019191, No. 3132019187 and No. 3132019327), China Postdoctoral Science Foundation (No.2019M651097 and No.2019M 651094) and Natural Science Foundation of Liaoning Province (No.2019-BS-026 and No.2019-ZD-0154).
- 1. Q.W. Shen, S.A. Li, G.G. Yang, J.L. Yuan, and N.B. Huang, J. Ceram. Process. Res. 20 (2019) 152-157.
- 2. Q.W. Shen, S.A. Li, G.G. Yang, S. Bengt, and J.L. Yuan, Energies, 12 (2019) 410-420.
- 3. Q.W. Shen, Y.D. Zhang, H. R. Ding, Y.Q. Xu, B.C. Shi, B.C. Y. Zheng, and J.L. Yuan, Energies, 10 (2017) 164-174.
-

- 4. Q.W. Shen, Y. Zheng, C. Luo, and C.G. Zheng, Chem. Eng. J. 225 (2014) 462-470.
-

- 5. Q.W. Shen, Y. Zheng, S.A. Li, H. R. Ding, Y.Q. Xu et al., J. Alloys Compd. 658 (2016) 125-131.
-

- 6. Q.W. Shen, L.N. Sun, and B.W. Wang, Int. J. Electrochem. Sci. 14 (2019) 1698-1712.
- 7. M. Siddique, S. K. Durrani, and E. Ahmed, J. Ceram. Process. Res. 16 (2015) 515-518.
- 8. H. W. Jin, J. H. Kim, Y. M. Park, C. Choi, H.J. Kim, and H. Kim, J. Ceram. Process. Res. 13(2012) 286-290.
- 9. H. Hashimoto, T. Kusunose, and T. Sekino, J. Ceram. Process. Res. 12(2011)223-227.
- 10. Q. Liao, Y.Chen, Y.Y.Wei, L.Y.Zhou, and H.H. Wang, Chem. Eng. J. 237(2014)146-152.
-

- 11. S. Boldrini, C. Mortalo, and S. Fasolin, Fuel Cells, 12(2012) 54-60.
-

- 12. J.Q. Zheng, Y.J. Zhu, and J.S. Xu, Mater. Lett. 100(2013)62-65.
-

- 13. Z. Yang, and Y.S. Lin, Ind. Eng. Chem. Res. 41 (2002) 2775-2784.
-

- 14. G. Guo, K. Lian, L. Wang, F. Gu, D. Han, and Z. Wang, RSC Adv. 4 (2014) 58699-58707.
-

- 15. Y. Wang, X. Yang, L. Lu, and X. Wang, Thermochim. Acta. 443 (2006) 225-230.
-

- 16. D. Tian, C. Zeng, H. Wang, X. Cheng, Y. Zheng, C. Xiang et al., Appl. Surf. Sci. 416 (2017) 547-564.
-

- 17. R. Zhang, Y. Wang, and R.C. Brown, Energy Convers. Manage. 48 (2007) 68-77.
-

- 18. T. Yamaguchi, Catal. Today 20 (1994) 199-217.
-

- 19. A.L. Kustov, O.P. Tkachenko, L.M. Kustov, and B.V. Romanovsky, Environ. Int. 37 (2011) 1053-1056.
-

- 20. B.C. Enger, R. Lødeng, and A. Holmen, Appl. Catal. A-Gen. 346 (2008) 1-27.
-

- 21. S. Pavlova, N. Sazonova, V. Sadykov, S. Pokrovskaya, V. Kuzmin, and G. Alikina, Catal. Today. 105 (2005) 367-371.
-

- 22. M. Alifanti, M. Florea, S. Somacescu, and V.I. Parvulescu, Appl. Catal. B-Environ. 60 (2005) 33-39.
-

- 23. R. Zhang, W. Yang, N. Luo, P. Li, Z. Lei, and B. Chen, Appl. Catal. B-Environ. 146 (2014) 94-104.
-

- 24. T.H. Shin, S. Ida, and T. Ishihara, J. Am. Chem. Soc. 133 (2011) 19399-19407.
-

 This Article
This Article
-
2020; 21(1): 64-68
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.64
- Received on Aug 20, 2019
- Revised on Nov 8, 2019
- Accepted on Nov 22, 2019
 Services
Services
- Abstract
introduction
materials and methods
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Qiuwan Shen
-
Marine Engineering College, Dalian Maritime University, Dalian, China
Tel : +86-13971559130, +86-13050561150 Fax: +0411-84728659 - E-mail: shenqiuwan@dlmu.edu.cn, yanggg@dlmu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.