- Characterization of some selected Ghanaian clay minerals for potential industrial applications
B. Onwona-Agyemana, N. Lyczkob, D. P. Minhb, A. Nzihoub and A. Yayaa,*
aDepartment of Materials Science & Engineering, School of Engineering Sciences, University of Ghana, Legon, Ghana
bUniversité de Toulouse, IMT Mines Albi, RAPSODEE CNRS UMR-5302, Campus Jarlard, F-81013 Albi cedex 09, France
The aim of this work was to
study five (5) selected local raw clay materials from Ghana using different
characterization techniques such as Thermogravimetric/Differential Thermal
Analysis (TG/DTA), X-ray diffraction (XRD), Fourier Transform Infra-red
Spectroscopy (FTIR), Scanning Electron Microscopy equipped with Energy
Dispersive X-ray Spectroscopy (SEM-EDX) and Nitrogen Desorption (Brunauer-Emmett-Teller, BET) specific surface
area analysis. The clay samples
studied are; Nkroful kaolin (NK), Amanfrom kaolin (AK), Ball clay (BC), Akyem
Feldspar (AF) and Akwatia silica (AS). SEM and EDX show the morphological
features of the five clay samples and also confirm the presence of some
dominant elemental compositions such as aluminium and silicon in all the
samples. FTIR show that the vibrations spectra in the region around 3,600-3,700
cm-1 and 700-800 cm-1 are due to M-OH groups and
that at 900-1000 cm-1 corresponds to Si-O-Si modes.
BET analysis gives specific surface area of the clay samples as NK (4.6 m2/g),
AK (21.9 m2/g), BC (25.50 m2/g), AS (0.79 m2/g)
and AF (0.49 m2/g). X-ray diffraction pattern
confirm the presence of quartz as the major reflection in all the samples
analysed and only kaolinite reflections appeared in three of the samples (NK,
AK and BC). All the kaolinite clays (NK, AK and BC) are suitable starting materials for the fabrication of
electroporcelain insulators, catalytic converters and diesel particulate
filters.
Keywords: Clay, Specific surface area, Electro-porcelain
Clay refers to the finest fraction of sediments that
consist of accumulations of different minerals such as quartz, feldspar and
many more which is formed by the weathering of silicate minerals in/on the
earth’s crust [1]. Clay has many vast benefits in medicine and
industrial purposes which has gained extensive research interest due
to its abundance and low cost [2]. In Africa, clay is mainly used in making
pots for storing water or food and earthen ware bowls for cooking.
Prehistoric practices such as geophagy, which is the
practice of ingesting earth materials or substances such as clay [3, 4] to
augment a scanty mineral deficient diet (for example; iron, copper, calcium,
zinc and manganese) [5], as part of a culture, or to stimulate a healing response
to sooth an infected and inflamed gastrointestinal lining [6] is still
prevalent in the 21st century.
Clays such as smectites, kaolinite and fibrous clay
minerals have been applied widely for drug delivery systems because of their
large specific surface area, pore volume and uniform porosity for sustained
release [7]. Smectites in particular are frequently used as substrate, because
it can retain large amounts of drug due to its cation exchange ability [7].
Others such as palygorskite, kaolinite and talc are extensively used in
pharmaceutical formulations because of their high specific
surface area, good rheological properties, chemical inertness,
low toxicity and good biocompatibility which is
highly suitable for patients [8]. The adsorptive properties of some clay
materials such as high pore volume, fine particles size and cation ion exchange
allows the removal of oils, toxins and contaminants from the skin, which makes
clay suitable for formulations in the
cosmetic industry [6, 9, 10]. Therapeutic uses of clay includes
mixing variable amounts of clay with different sea or salt lake mineral waters
to form pastes for fighting chronic rheumatism and bone muscle diseases
[9, 11-13]. Many studies have proven that clays can be used for
biomaterials and other medical devices such as biosensors [14-18]. In
agriculture, sepiolite and palygorskite clay suspensions have been used as a
tool to reduce the amount of cadmium (Cd) concentration in the soil. This was
done to solve the pollution of soil by this heavy metal. It has also been used
as fertilizer because of its nutrients content, thereby increasing crop yield
[19].
Kaolin is widely used in the paper industry to coat the
surface of paper for brighter colors, as filler in many composite
materials to add strength and to improve the abrasion resistance, and rigidity
to both natural and synthetic rubber products at a low cost. It is also used in
the ceramic industry as an insulator and paint industry due to its high
covering power, low cost and high resistance to chemical attack [20-24].
Recently, research has shown that archeological clay
obtained in Komaland, from the Northern Region of Ghana has prospects to
enhance human fetal osteoblast cells growth in vitro [25]. Also, other
research has shown that treated kaolin from natural clay using chemical and
thermal reactions inhibit Hela cervical cancer cells in vitro [26]. Some
modified clay minerals with lidocaine and silver have been exploited in burn
wounds and antisepsis respectively [27]. In Africa, several kaolin deposits are
not utilized effectively and may serve as assets in economic gains and research
opportunities [28]. Natural clay has been inadequately exploited to know their
chemical composition, their characteristics and applications as compared to
those that are modified, synthetic or refined. In Ghana, although the clay
industry is huge, the properties that inform their usage are limited.
This work seeks to explore various clay deposits from five
different geographical locations, that is; Amanfrom Kaolin (AK),
Ball clay (BC), Nkronful Kaolin (NK), Akyem Feldspar (AF), and Akwatia silica
(AS) and characterize them using techniques such as TGA/DSC, XRD, FTIR, SEM
equipped with EDX and BET so as to better understand their
characteristics for future industrial applications such as electroporcelain insulators, diesel particulate filters and as washcoat materials in catalytic
converters.
The raw materials for this
study were collected from kaolin deposits in Ghana located in the Western and
Central regions.
Powder samples were prepared for this study using a 400 g
quantity of lumpy kaolin deposits, Nkroful and Amanfrom, which were first
ground using a Thomas grinding machine to break up the agglomerates. The samples
were each further milled for 13 h in a cascading ball mill
using alumina balls to obtain fine powders. These were
sieved through a gradient sieve with aperture size of 63 μm.
The specific surface area of the clay materials was determined
using BET method with a MICROMERITICS ASAP 2010 apparatus.
Fourier transformed infra-red spectroscopy (FTIR) was carried
out with a Shimadzu spectrophotometer (FTIR-8400S) scanning between 4,000 and
500 cm−1. Each clay sample was finely ground in a mortar and then
mixed with potassium bromide (KBr) powder. The powder mixture was put in a
mould and pressed at high pressure to form thin pellets. In order to minimize
the amount of water adsorbed, the pellets were heated in a furnace overnight at
130 ºC.
XRD analyses were performed using a Philips diffractometer
(PANalytical, X’pert Pro MPD model) with a Bragg-Brentano configuration with
voltage of 45 kV and current of 40 mA. The measurement was done at room
temperature using a filtered Cu Kα (λ = 0.15418 nm) radiation and scanning from
10 to 70 degrees with scan speed of 0.042°/sec.
The morphology and microchemical features of the clay
samples were observed with a Field Emission Scanning
Electron Microscope (FE-SEM Philips XL30) equipped
with an energy dispersive x-ray spectroscope. Particles images were obtained
with a secondary detector.
Thermal analyses were carried out with a TGA/DSC apparatus,
SDT Q600 by TA Instruments. 15 mg of solid samples were
heated over the temperature range from ambient to 1,000 oC at a
heating rate of 5 oC/min in air atmosphere with a 100 mL·min-1
flow rate. Samples were analyzed in alumina crucibles and the reference was an
empty alumina pan.
The results for the TGA/DSC curves for all the samples
(AK, BC, & NK, AF, and AS) are given in Fig. 1 for the temperature range
0-1000 oC. As evident in the TGA/DSC peaks, there is some
weight losses observed in the samples.
For samples AK & BC,
weight loss occurred in the temperature
ranges of 200-700 oC which are 2.5%, 5.9% & 3.3%, 5.4%
respectively. After 700 oC, the weight loss in AK and BC are
0.5% and 0.4% corresponding to total weight
loss of 8.9% & 9.1% respectively. The difference in mass loss between the
two samples is about 0.2% which is an indication of similar heating pattern
(i.e, AK & BC) as seen in their TGA/DSC curves, while NK gave a total mass
loss of 6.1% at 700 oC. The endothermic peak at 200 oC
indicated the removal of water and other hydroxylated functional groups
attached to the clay minerals. As temperature
is increased to 700 oC, α-β quartz transition occurs without further mass loss which
is in agreement with earlier reports for quartz transitions [29]. For AS and
AF, total weight losses are relatively insignificant compare to the three
kaolinite materials. Additionally, there appears to be an exothermic peak in
all the samples at 980 oC and this is attributed to the
formation of a spinel phase at higher temperatures [17, 26].
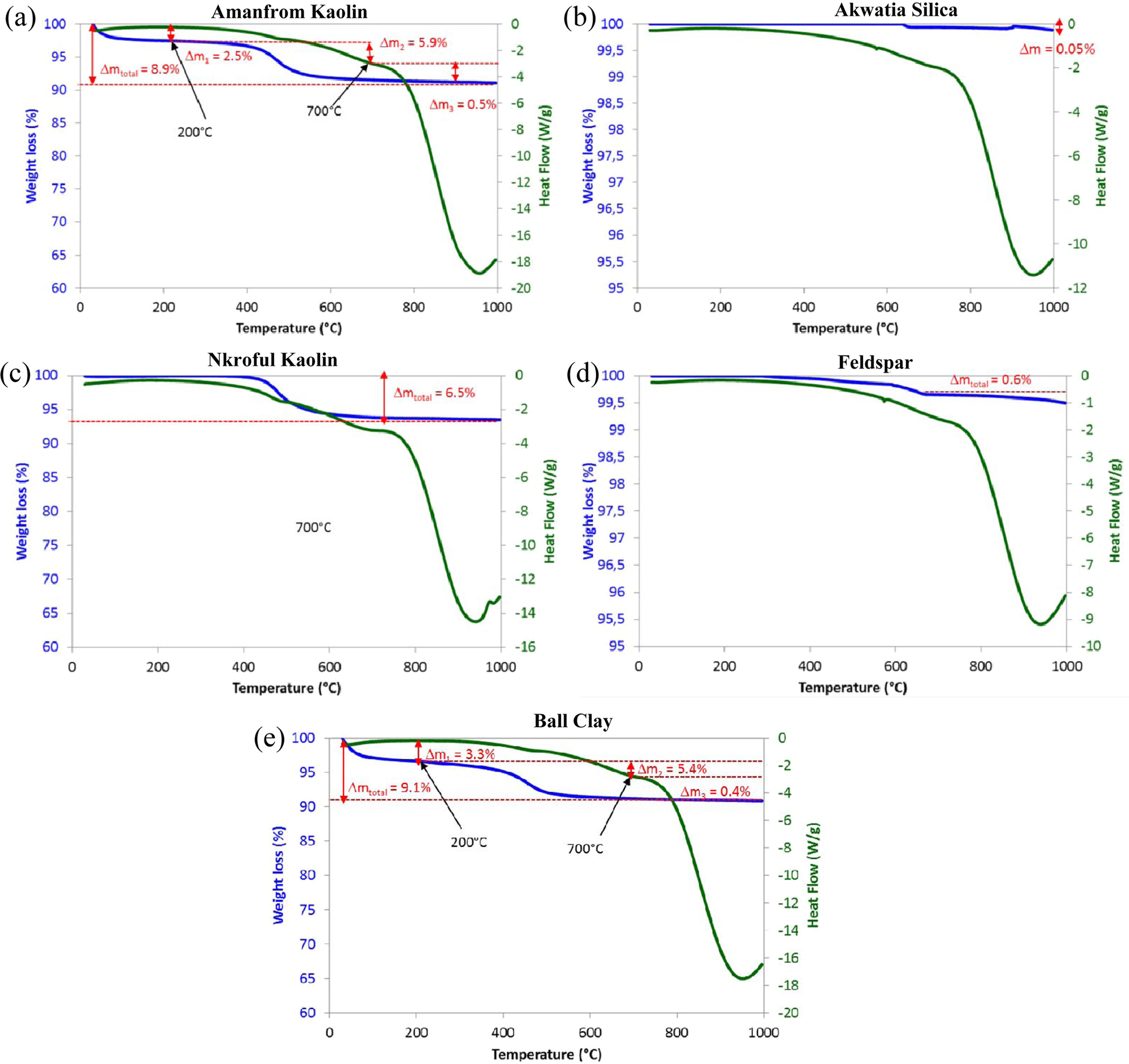
|
Fig. 1 TGA/DSC curves for (a) Amanfrom kaolin, (b) Akawtia silica, (c) Nkroful Kaolin, (d) Akyem-Akroso Feldspar and (e) Ball Clay. |
FTIR spectroscopy has been used extensively to
characterize clay and clay based minerals [30, 31]. Fig. 2 is the FTIR for
the various samples used in this study. The stretching and bending vibrations
found in the region of 3,600-3,700 cm-1
and 700-800 cm-1
respectively are due to M-OH groups [32]. Also, the weak band around
1,600-1,631 cm-1
is ascribed to the bending modes of physisorbed water molecules with other OH
groups present in the clay minerals. These stretching and bending vibrations
occurs in all the kaolinites (AK, NK & BC) but not observed in the
silica-based, AS and AF as shown in Fig. 2. The bands around
900-1,000 cm-1
are attributed to Si-O-Si stretching vibrations, see Fig. 2.
Additionally, the appearance of medium intensity bands around
500-650 cm-1 is
attributed to the presence of quartz in all the samples,
(showing intense band for AK and AF). The sharp peaks observed in the spectra
for NK & AK, suggest a well order kaolinite phases in these two clay
samples. The FTIR analysis closely agrees with the studies of S. Mahmoudi et
al. on clay materials from Cameroon [33].
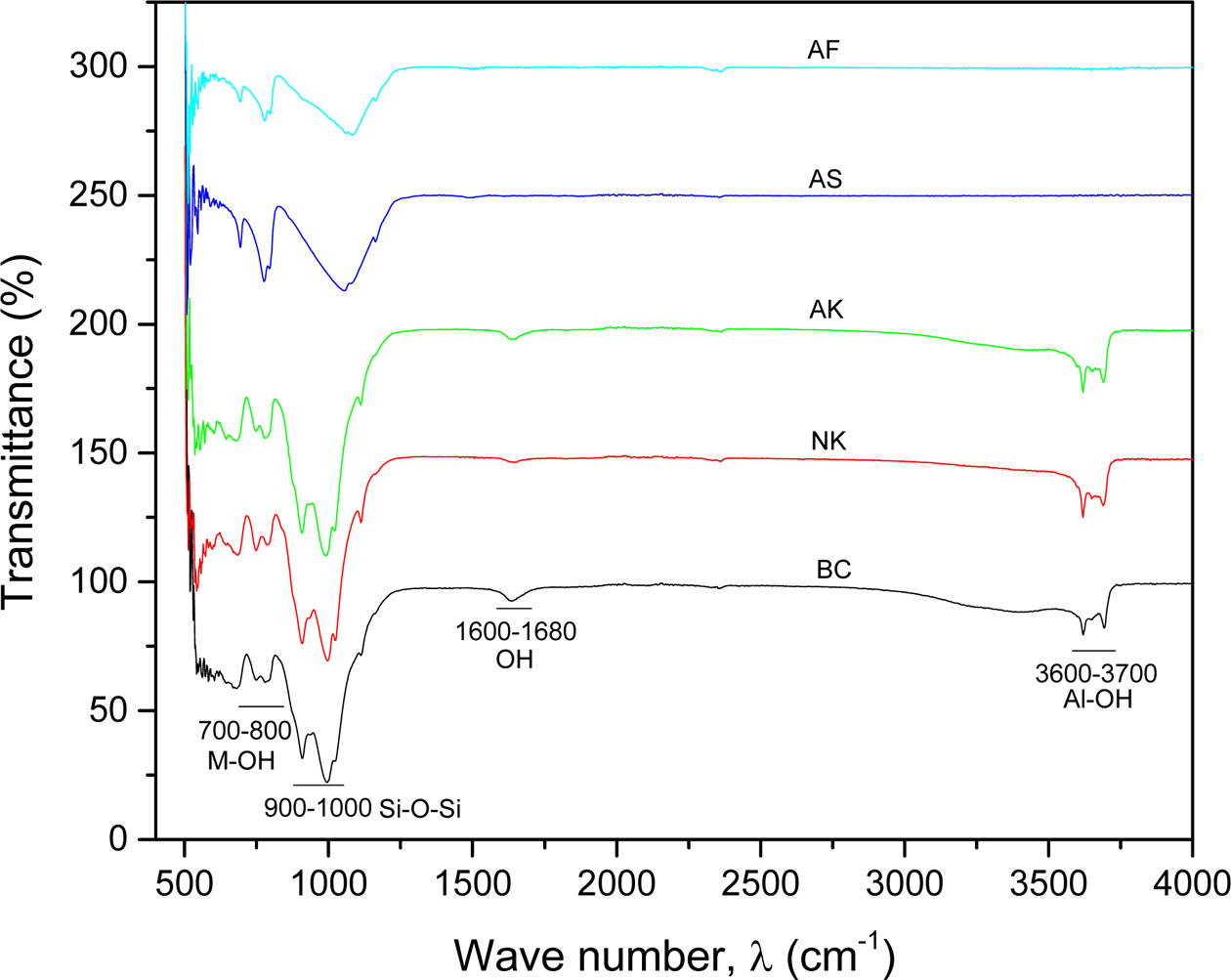
|
Fig. 2 FTIR analysis for Ball clay (BC), Nkroful (NK), Amanfrom (AK), Akwatia Silica (AS) and Akyem Feldspar (AF). |
The minerals structure in
clays can successfully be determined through the use of X-ray diffraction. This
can also be used to validate FTIR analysis. XRD was used to identify the
structural phases present in the samples. The XRD patterns for the AS and AF are
very similar with the dominant phase present being SiO2, whereas that of NK, AK and BC
contains Al2Si2O5(OH)4 and Na (Al Si3 O8)
as major components in addition to some traces of SiO2 as shown in
Fig. 3 and also in Table 1. Furthermore, AF was found to contain some traces of
potassium, hydrogen and sulphur suggesting that, it is a potash feldspar (see
Table 1). This also goes to confirm the identification of the stretching
frequencies observed in the FTIR in
which similar vibrations frequencies were found for the three kaolinite groups
at about 3,600-3,700 cm-1 and that of the silica and
feldspar also having same frequency
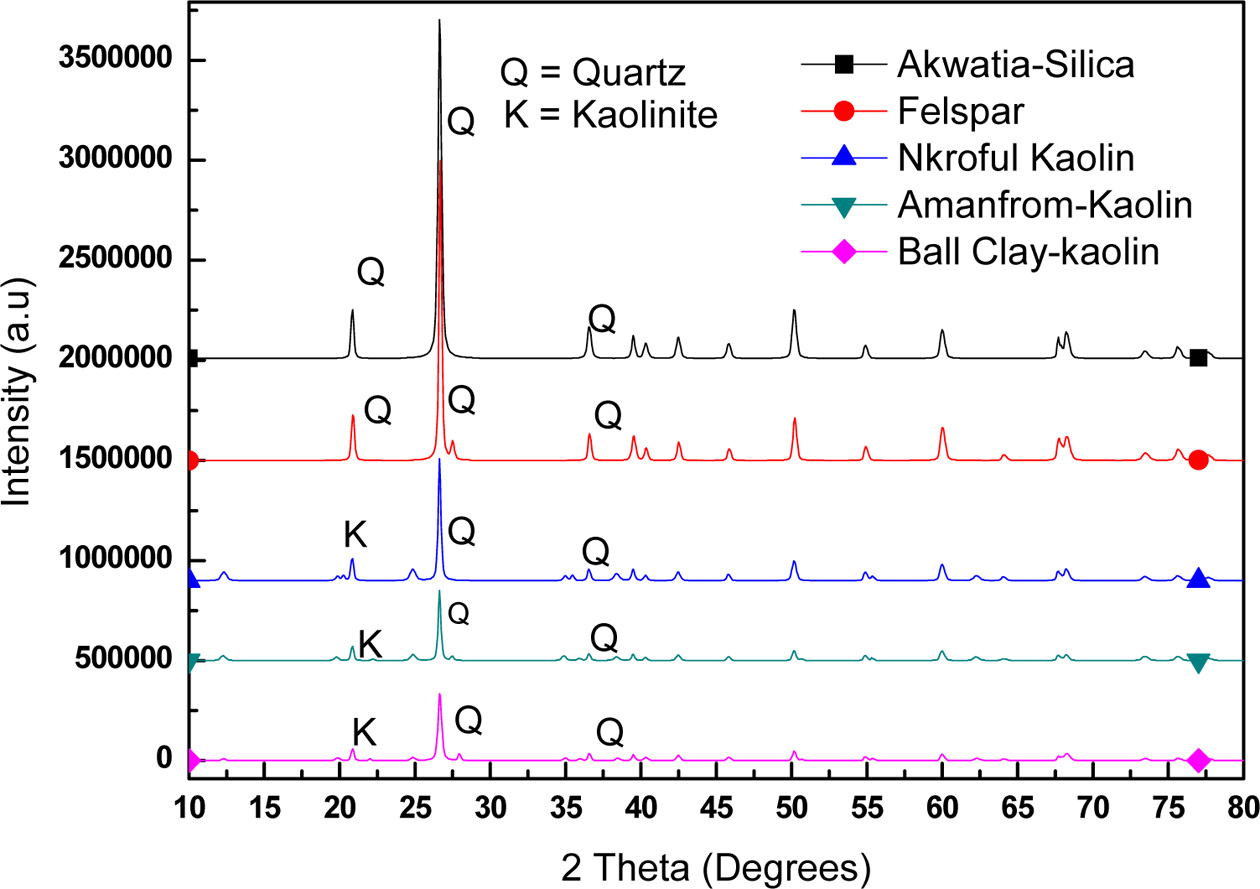
|
Fig. 3 X-ray diffraction patterns of the various samples. |
|
Table 1 Identified crystalline phases from X-ray diffraction studies on the five (5) clay samples. |
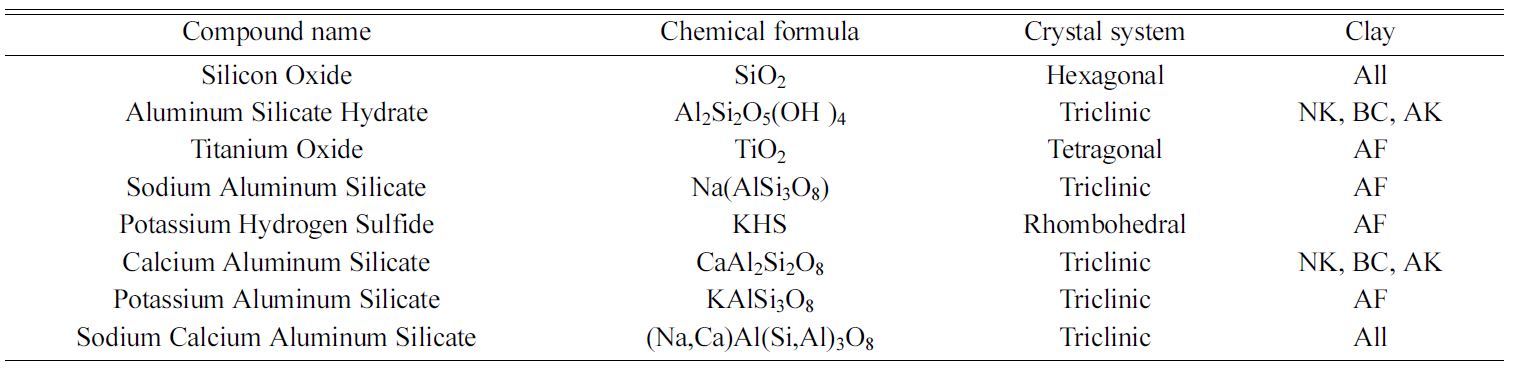
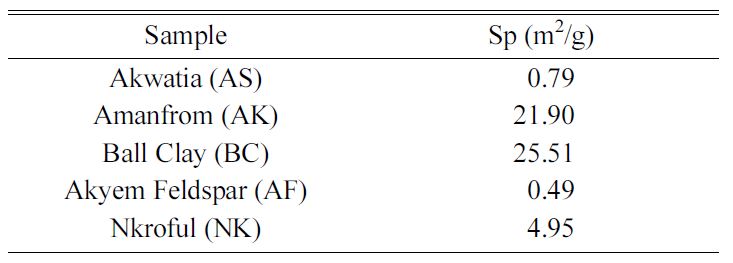
Table 2 shows the BET specific surface area measure-ments for the
various samples. It is clear that, BC has the highest surface area of 25.51 m2/g
which explains why it can absorb large amount of water making it plastic
because of large pore volumes and its finer particle sizes. Similarly, AK has
surface area of about 21.90 m2/g but is not as plastic as ball clay.
Nkroful kaolin has a surface area of 4.9 m2/g making it the least of
the kaolinite groups which becomes hard when dry. The other silica-based have
the least surface area; AF with 0.49 and AS 0.79 m2/g respectively,
which have nearly similar pores and show why they are non-absorbers
and have a larger particle size than the others.
The high specific surface area
of BC and AK can be applied in the areas of wash-coat materials for catalytic converters used in purifying
exhaust gasses from internal combustion engines [30]. The catalyst wash-coat is a
carrier for the catalytic materials, which is used to disperse catalyst
materials over a high surface area. The catalytic materials are suspended in
the washcoat before application to the core body and the washcoat have rough,
irregular surface to increase surface area, which helps to maximize the catalytically active
surface available to react with the
engine exhaust gasses.
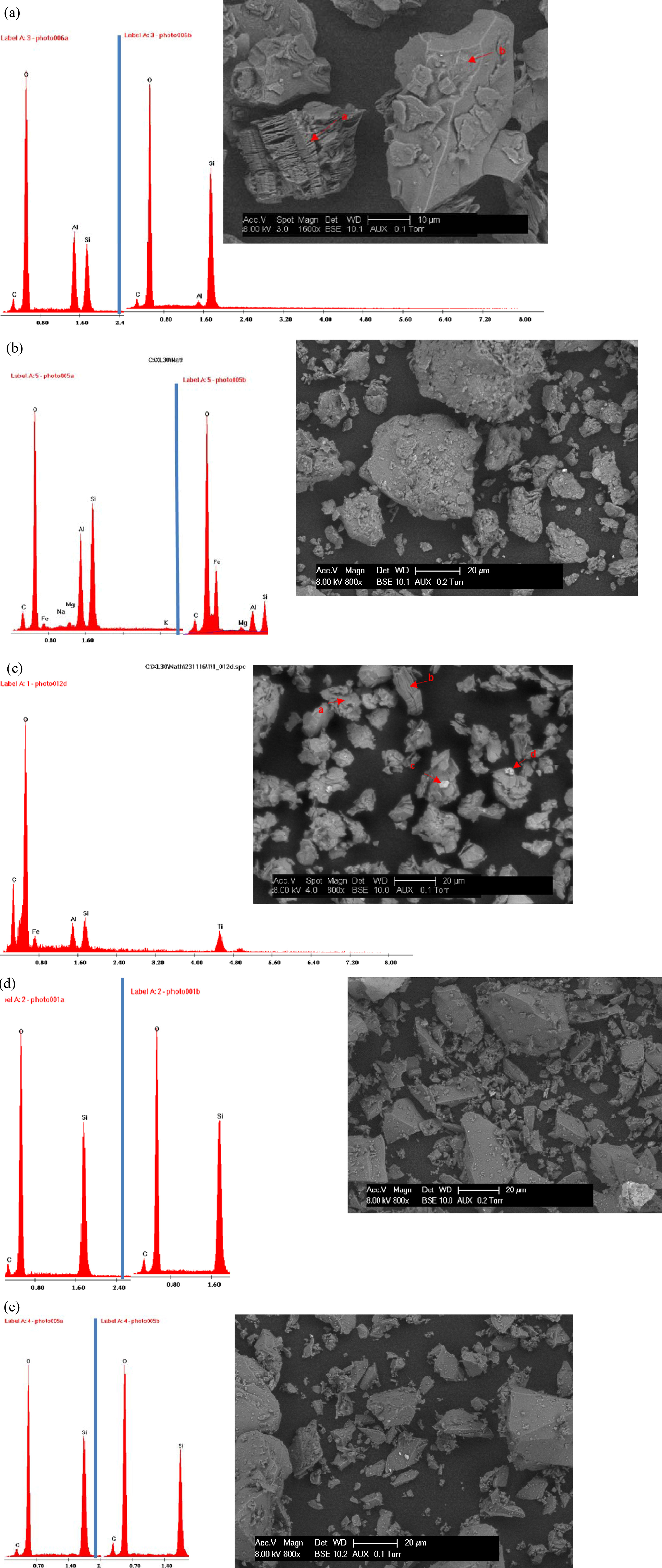
The SEM images and EDX analysis of the samples are given
in Fig. 4. Morphological features show that, NK is made up of well-developed
kaolinite crystals with layered morphology (Fig. 4a). For AK and BC, both show
a relatively porous aggregate morphology. The porous aggregates consist of
stacks of hexagonal kaolinites plates and some individual crystals (Fig. 4b-c).
The EDX analysis of the various peaks also reveals Si & Al as the dominant
elements with some traces of other elements which agrees with the FTIR and XRD
studies reported earlier. On the other hand, AS and AF show a platelet crystals
with their characteristic EDS spectra (see Fig. 4d-e) giving Si as the most
dominant elements which is in agreement with the FTIR and XRD studies. The high
silica content in AS and AF can be exploited by heating the samples in the
presence of a carbon source in an inert or vacuum chamber at high temperatures
to form silicon carbide [31]. Since SiC has good oxidation, wear
resistance, high hardness and thermal stability it can be used as protective
coatings on ceramic components in the aerospace industry [32] and as diesel
particulate filter [33].
|
Fig. 4 Scanning electron microscopy images with resolution of 20 μm and EDX spectra for; (a) Nkroful kaolin, (b) Ball clay, (c) Amanfrom, (d) Akwetia silica and (e) Akyem feldspar. |
In this work five types of Ghanaian clays have been evaluated
for their potentials in; diesel particulate filters, electroporcelain
insulators and catalytic converters. Different characterization
techniques such as XRD, SEM equipped with EDX, BET, FTIR and TG-DTA were used
extensively to study the clay samples from different locations in Ghana. XRD
patterns showed the presence of quartz as the dominant phase in all the samples
examined whilst the EDX confirmed the presence of silicon and
aluminium as the major elemental compositions. These
characterisations have revealed very important features of these minerals that
could be tailored towards specific industrial applications.
All authors declare no conflicts of interest in this
paper.
- 1. A. Yaya, E.K. Tiburu, M.E.Vickers, J.K. Efavi, B. Onwona-Agyeman, and K.W. Knowles, Appl. Clay Sci. 150 (2017)125-130.
-

- 2. J.H. Choy, S.J. Choi, J.M. Oh andTaeun Park, Appl. Clay Sci. 36 (2006) 122-132.
-

- 3. M.J.Wilson, J. Chem. Ecol. 29 (2003)1525-1547.
-

- 4. R.E. Ferrell, Clays Clay Miner. 56(2008) 751-760.
-

- 5. J.M. Hunter, Geogr. Rev. 63 (1973)170-1953.
-

- 6. M.I. Carretero, Appl. Clay Sci. 21(2002) 155-163.
-

- 7. R.B. Asamoah, E. Nyankson, E.Annan, B. Agyei-Tuffour, J.K. Efavi, K. Kan-Dapaah, V.A. Apalangya, L.N.W.Damoah, D. Dodoo-Arhin, E.K. Tiburu,S.K. Kwofie, B. Onwona-Agyeman, and A. Yaya, Orient. J. Chem. 34 (2018) 4.
-

- 8. C. Aguzzi, P. Cerezo, C.Viseras, and C. Caramella, Appl. Clay Sci. 36 (2007) 22-36.
-

- 9. M.I. Carretero and G. Lagaly,Appl. Clay Sci. 36 (2007) 1-3.
-

- 10. C.S.F. Gomes and J.B.P. Silva,Appl. Clay Sci. 36 (2007) 4-21.
-

- 11. F. Tateo and V. Summa, Appl. Clay Sci. 36 (2007) 64-76.
-

- 12. C. Viseras, C. Aguzzi, P.Cerezo et al., Appl. Clay Sci. 36 (2007) 37-50.
-

- 13. F. Veniale, A. Bettero, P.G.Jobstraibizer et al., Appl. Clay Sci. 36 (2007)141-147.
-

- 14. M. Ghadiri, W. Chrzanowski,and R. Rohanizadeh., Roy. Soc. Chem. Adv. 5 (2015) 29467-29481.
-

- 15. N. An, C. Hui, X. Yu et al.,Appl. Clay Sci. 114 (2015) 283-296.
-

- 16. I. Aksu, E. Bazilevskaya, andZ. T. Karpyn, Geo. Res. J. 7 (2015) 1-13.
-

- 17. M.I. Carretero and M. Pozo,Appl. Clay Sci. 46 (2009) 73-80.
-

- 18. M.H. Kim, G. Choi, A.Elzatahry, A. Vinu, Y. Bin Choy, and Jin-Ho Choy, Clay and Clay Minerals 64[2]2016 115-130.
-

- 19. X. Liang, J. Han, and Y. Xu,Geoderma, 235-236 (2014) 9-18.
-

- 20. H.H Murray, Clays, and ClayMinerals 10[1] 1961 291-298.
-

- 21. H.H. Murray, Appl. Clay Sci.17 (2000) 207-221.
-

- 22. H. Celik, Appl. Clay Sci. 50(2010) 245-254.
-

- 23. P. Melgarejo, J.J. Martínez,F. Hernández, R. Martı́nez-Font, P. Barrows, and A. Erez,Sci. Hortic. (Amsterdam) 100 (2004) 349-353.
-

- 24. C. Volzone, Appl. Clay Sci. 36(2007) 191-196.
-

- 25. E.K. Tiburu, B.W. Kankpeyeng,S.N. Nkumbaan, A. Salifu, and J. Q. Zhuang, J. Biomimetics, Biomater. Biomed.Eng. 30 (2017) 45-60.
-

- 26. E. Annan, B. Agyei-Tuffour, Y.D. Bensah, D.S. Konadu, A. Yaya, B. Onwona-Agyeman, and E. Nyankson, CogentEngineering 5 (2018) 1476017. https://doi.org/10.1080/23311916.2018.1476017.
-

- 27. J. Santaren, E. Ruiz-hitzky,P. Aranda, A. Antonio, and A. Esteban, Development in Clay Science 3 (2011)393-452.
-

- 28. G.I.E. Ekosse, Appl. Clay Sci.50 (2010) 212-236.
-

- 29. A.N. Duowona, E. Nyankson J.K.Efavi, L.N.Damoah, E. Tiburu, D.Dodoo-Arhin, V. Apalangya, T. Tomychiek, and A.Yaya, Journal of Ceramic Processing Research 19[2] (2018) 95-100.
- 30. B. Onwona-Agyeman, R. Suzuki,H. Yano, and H. Tomoda, SAE Int. J. Engines 1[1] (2008) 1341-1346.
-

- 31. P.D. Miller, J.G. Lee, and I.B. Cutter, J. Am. Cer. Soc. 62[3-4] (1979) 147-149.
-

- 32. B.S. Sung and Y. H. Yun,Advances in Materials Science and Engineering (2017).
-

- 33. A. Itoh, K. Shimato, T. Komoriet al., SAE Transactions 102[3](1993) 505-513.
 This Article
This Article
-
2020; 21(1): 35-41
Published on Feb 28, 2020
- 10.36410/jcpr.2020.21.1.35
- Received on Jul 12, 2019
- Revised on Nov 18, 2019
- Accepted on Nov 22, 2019
 Services
Services
- Abstract
introduction
materials and methodology
results and discussions
conclusion
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- A. Yaya
-
Department of Materials Science & Engineering, School of Engineering Sciences, University of Ghana, Legon, Ghana
Tel : +233559278551 - E-mail: ayaya@ug.edu.gh






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.