- Roles of talc-illite on phase transformation, vitrification and physical properties of a triaxial porcelain body
Suthee Wattanasiriwecha,b and Darunee Wattanasiriwecha,b,*
aCenter of Innovative Materials for Sustainability, School of Science, Mae Fah Luang University, Thailand
bCircular Economy for Waste-free Thailand Research Group, Mae Fah Luang University, Thailand
Flux forming additives have
played an important role of energy reduction in the ceramic industry for
centuries. Though there are available fluxing materials in the market, attempts
of searching for new flux systems have still been extensively done. This paper
presents the use of a combined flux system and its roles on phase
transformation and physical properties of a triaxial porcelain body. Illite was
used as the primary flux in the main recipe while talc, the supplementary flux,
was added at 0, 3, 5 weight %. In pure form, talc dissociated into enstatite
around 900 oC but when mixed in the recipe, phase changes of
talc differed. Talc and illite disappeared around 1,000 oC.
Around 1,200-1,300 oC, indialite and pyrophillite appeared only
on the body with talc addition. Early densification could be observed when talc
was present, suggesting that talc also played an important role in
vitrification. The body with 3% talc showed the greatest firing shrinkage and
flexural strength but lowest water absorption after firing at 1,200 oC,
which was ~50 oC lower than the body without talc. Further
increasing of talc content to 5% resulted in an adverse effect of these
properties.
Keywords: Triaxial porcelain, Illite, Talc, Phase transformation, Vitrification
Porcelains are vitreous ceramic whitewares which are used
extensively in table wares, dental prosthetics, and decorative wares. With the
room temperature resistivity between 1012-1014
Ω, porcelain ceramics are extensively used as an insulating material
[1].
Porcelains typically have a triaxial formulation
comprising of clay-feldspar-quartz as the main ingredients.
Quartz is partially added to reduce pyroplastic deformation
[2].
Due to the high firing temperature (>1,200 oC
for soft porcelain and 1,400 oC for hard porcelain),
flux-forming additives were generally employed in order to reduce
the energy consumption without compromising productivity and product
[3-6].
Some of the reported works are exemplified
here. Das compared densification behavior of K-and Na-feldspar-containing
porcelain bodies and found that Na-feldspar containing body exhibited maximum
densification rate at 1,171 oC
compared to 1,195 oC for the K-feldspar containing
body [7].
The Na-feldspar containing body achieved higher density,
lower water absorption and highest flexural strength at 1,200 oC.
Dana et al. have reported the enhancement in densification and
strength of triaxial porcelain when B.F. slag was used in substitution
of potash feldspar [8].
The addition of slag resulted in the diminishment of
quartz and mullite needles but development of anorthite phase (CaO·Al2O3·2SiO2).
Bragança and Berman showed that the glass-powdered porcelain in which feldspar
was replaced by recycled soda-lime glass sintered at 100 oC
lower than the traditional porcelain [9].
However, when fired at the proper
temperature, traditional porcelain exhibited higher modulus of
rupture due to the presence of secondary mullite crystals. Combined flux system
was typically used along with the aids of muscovite mineral [10].
Pyroplastic deformation, which is related to properties
of liquid phases formed during firing, was found to be influenced by ratio of
the key ions used in the formula. It was reported that that a definite
combination at SiO2/Al2O3 ratio of 5 and Na2O/K2O
ratio of 4 gave the lowest pyroplastic deformation in the porcelain body
formulations [11].
Some of the reported literatures proposed an alternative
method in energy consumption reduction such as direct sintering method whose total
processing time was reduced by a factor of
50% and the sintering temperature was
decreased from 1,200 oC to 1,175 oC [12].
In this scenario, sample was delivered
directly to the furnace pre-set at the
sintering temperatures. The samples were held for 15 min
before cooling down to 700 oC at a rate of 30 oC/min
and followed by cooling to room temperature. The use of
microwave sintering was reported to reduce sintering temperature by ~75oC
and reduce dwell time from 15 min to 5 min while retaining
comparable physical properties [13].
As previously discussed, the irreversible thermal expansion
of ceramic bodies containing ball clay started around
900 oC [14].
Most ball clay contains illite, which
is a mica- type mineral with its high potassium content in its structure, and
thus fusibility. The structure of illite is composed of two tetrahedral sheets
sandwiched to an octahedral sheet to form a 2:1 layer phyllosilicate. Illite
serves as an excellent flux because of its small particle size and being a part
of body matrix. The body porosity was, thus, reduced by the
onset of melting and the bodies began to shrink at a temperature as
low as 900 oC [14].
This result was in good agreement
with a study by Aras [15],
who used illite in substitution of kaolin. It was shown
that the high K content in illite caused a large amount of
liquids and the reduction of mullite formation temperature.
When illitic clay was fired, illite structure first collapsed around
900-950 oC resulting in liquid phase
formation [16, 17].
Another study using an in-situ high
temperature X-ray diffraction showed that transition from
illite phase to dehydroxylated phase started around 525 oC
in static air and higher at about 550 oC in vacuum.
Phase transformation from dehydroxylated to mullite occurred at above
1,100 oC [18].
It was also pointed out that transformation could be
affected by sample’s properties such as particle size, crystallinity, and
vacant type [19].
Illite was reported to gradually diminish at 600 oC
before completely disappearing at 800 oC upon firing
[19].
Our previous attempt to use illite as the primary flux
in place of a potash feldspar in a triaxial porcelain body was achieved and the
firing temperature could be lowered up to 100 oC [20].
Talc is a hydrated magnesium sheet silicate with the chemical
formula Mg3Si4O10(OH)2. Talc has a
2:1 structure with an octahedral brucite sandwiched between
two tetrahedral silicate layers [21].
Talc is typically used as a filler in composite
materials to decrease the production costs while improving the physical and chemical
properties and also providing new functionalities [22].
From literatures, talc progressively lost its hydroxyl groups
at above 900 oC and re-crystallized into different
forms of enstatite (anhydrous magnesium silicate) above
1,050 oC before melting at 1,500 oC [23].
The use of talc with other fluxes e.g.
nepheline syenite has been reported [24].
Appropriate amount of talc added to the stoneware
body was reported to give optimum properties and decrease
sintering temperature [25].
When feldspar was partly replaced by talc, the body
fired at high temperature contained more liquid of lower viscosity than
the standard body containing only feldspar flux [26].
In this study, our further
attempt to reduce the firing temperature with the aids of talc in the body
containing illite as the primary flux was performed. Phase changes and physical
properties were determined and discussed here.
China clay whose main minerals were halloysite and kaolinite
was received from Ranong province, Thailand. Quartz and
talc (Mg3Si4O10(OH)2) were
purchased from a domestic commercial supplier. The illite mineral (KyAl4Si8-yAlyO20(OH)4)
where y = 1-1.5, Green shale) was received from Ward’s Natural Science,
Rochester, New York, USA. Analysis of illite and china clay was previously
reported [20].
Analysis of talc was assessed using
standard protocols, X-ray diffraction (XRD), scanning electron
microscopy (SEM), and size analysis using laser diffraction. The lateral size
(a) of plate-like talc particle was analyzed based on the method described by
Pérez-Maqueda et al. as shown in Eq. (1) [27]: 
d001 (4.67 nm or
4.67×10-9
m) was determined from the broadening of XRD peak.
esd is the equivalent spherical diameter
(m) which can be calculated from Eq. (2) as follows;
where S is the specific surface
area (6.02 m2·g-1) determined
using the BET technique and r
is the density (2.8 kg·m-3).
The body recipe contained 50 weight % china clay, 25
weight % quartz and 25 weight % illite. Talc (0, 3, 5 weight %) was finally
added and the samples were denoted as IT0, IT3 and IT5
respectively. Details of experiments were also previously addressed [20].
Summary of the experiment is given shortly here. The
starting materials were mixed, ground using a ball milling technique and sieved
through a 325-mesh sieve prior to drying in an oven overnight. The dried clay
was then pressed at a compaction pressure of 50 MPa into a rectangular bar with
the dimensions of 5 mm ´ 5
mm ´ 60 mm. Each
set of the test samples was heated in the range of 900-1,300 oC
in air atmosphere. Water absorption of the fired samples was examined based on the
Archimedes’ principle with the details of experiment reported
in ref [28].
The flexural strength was
measured using a Universal Testing Machine (Instron 2000, UK)
by the three-point bending technique. For all
measurements, the support span of 80 mm
and the crosshead speed of 5 mm/min were selected. The flexural strength was
measured using the formulae s = 3/2 [(FL)/bd2],
where is the flexural strength, F is the load at the fracture point (N), L is
the span length; b and d are the width and thickness of the specimens
respectively. These values, which were averaged from five samples, were plotted as a function of
firing temperature. Phase analysis was observed using an X-ray diffraction (XRD)
technique equipped with the software
X’pert High Score Plus (X’pert Pro MPD, Philips, Netherlands).
Microstructural development of the fired bars
was examined with scanning electron microscope (SEM: LEO 1450 VP). The fired
test bars were etched with 4% hydrofluoric acid (HF) before the microscopical
examination.
Analysis of phase changes, chemical contents and particle
size of clay and illite were reported elsewhere [20]
so only analysis of
talc is addressed in this paper. Lateral size of the talc was calculated according
to Eq. (1) was 2.19 mm. Morphological observation using an SEM of the talc used
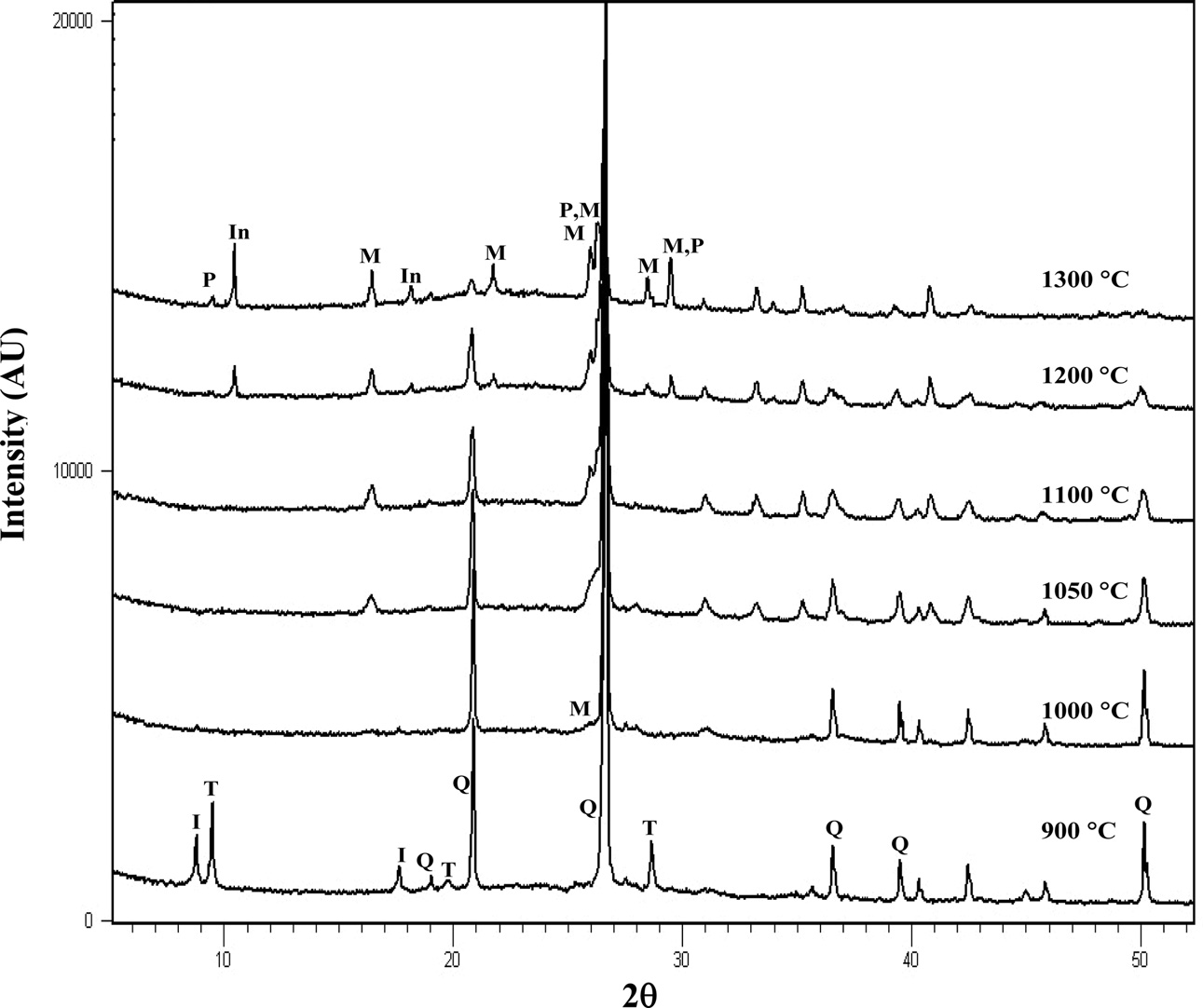
in this study is shown in Fig. 1. X-ray diffraction (XRD) analysis (Fig. 2)
shows that talc mainly contained talc mineral with a small amount of quartz and
magnesite (MgCO3) as well as a small trace of dolomite (CaCO3·MgCO3).
Upon firing to 800 oC, talc reflections started to diminish.
Reflections of enstatite (MgSiO3) started to appear around 900 oC.
This result was in good agreement with the report by Sabouang et al. [29].
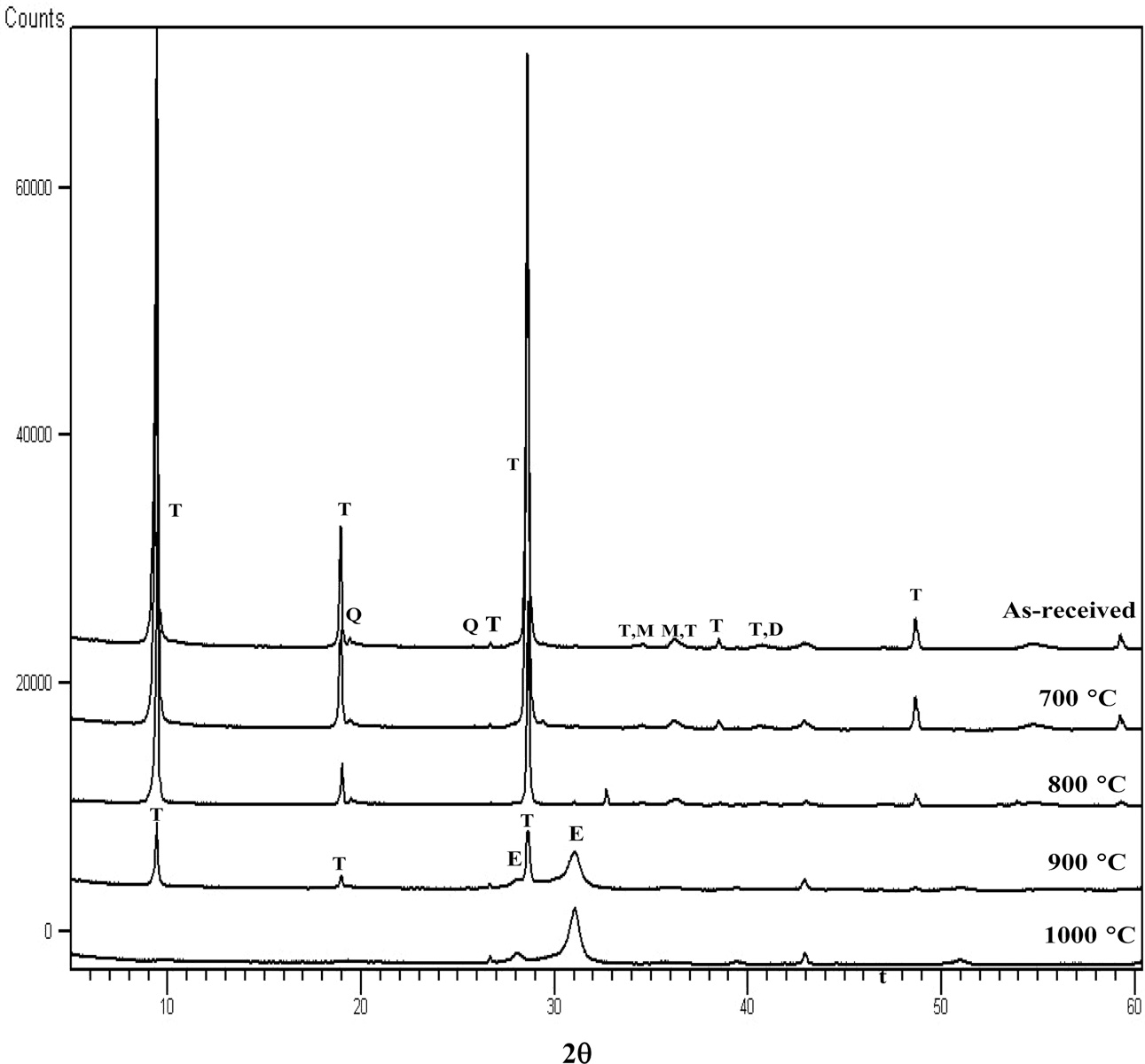
XRD spectra of the fired sample bars are displayed in Fig.
3. Only those with 5% talc are shown since the samples with less percentage of
talc had relatively similar reactions upon firing but with lesser extensity of
the phase compositions due to the lesser content of talc. At
900 oC, reflections belonging to quartz, illite and talc
existed. It was noted that the reflections belonging to enstatite, which
appeared when pure talc was fired, were not observed in the spectra. At
1,000 oC, talc and illite reflections disappeared while mullite
started to emerge and got stronger in intensity with the increase of firing
temperatures. Primary mullite content was reported to
slightly increase with increased sintering temperature through
transformation mullitization process of kaolinite [28]
. At 1,200-1,300 oC, two types of other
aluminosilicate minerals, indialite and pyrophyllite, appeared.
Indialite (Mg2Al4Si5O18), with a
chemical formula similar to cordierite, had a hexagonal structure so it was
once thought to be cordierite appeared at these temperatures. However, the work
done by Miyashiro showed that cordierite had a triplet around 29-30 o2q, while indialite only showed a
single reflection at this region [30].
Some recent literatures, however, stated that
indialite is a high temperature form of cordierite [31, 32].
The reflection at 9.5 o2q could be indexed as pyrophyllite (Al2Si4O4(OH)2)
or talc (X’pert High Score Plus: reference number
00-003-0170 and 00-003-0881 respectively) due to the close peak positions of
these two compounds at this angle. Pyrophyllite is a 2:1 clay mineral without
interlayer cation similar to talc [33]
. If this reflection truly belonged to talc, it would be
puzzling as to how it reappeared. Re-examination on another sample gave the
same result, suggesting that this observation was reliable. Neither enstatite
nor dehydrated talc, which appeared in the heat-treated pure talc sample, was
observed in this set of samples. It is noted that indialite and pyrophyllite
(or talc) reflections were not observed in the IT0 samples.
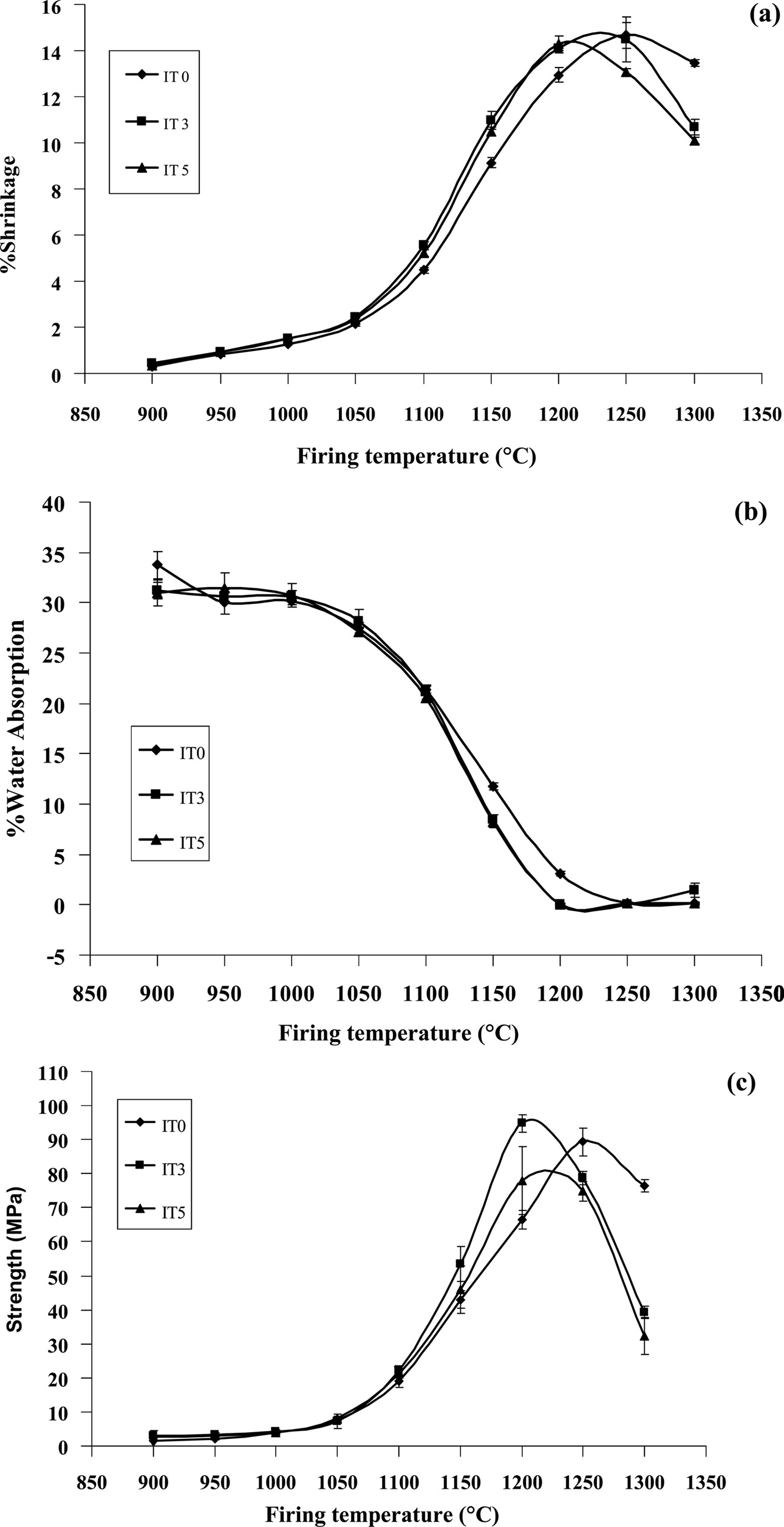
Key physical properties of the samples such as firing
shrinkage and water absorption as well as mechanical properties
are shown in Fig. 4. The IT0 samples showed the greatest
shrinkage at 1250 oC while the IT3 and IT5 samples
showed the greatest shrinkage at around 1,200-1,250 oC and
1,200 oC respectively (Fig. 4(a)). All the samples showed
expansion due to expansion of trapped gas (bloating) after reaching
the greatest shrinkage. Trapped air in a ceramic body could be caused
by arise due to over firing, too rapid firing, or the increase in the content
of volatile materials such as manganese oxide and iron ochre [34].
In our present study, it was believed that over firing
was the reason for bloating as also suggested by the finding by López and
Rodríguez [35].
Water absorption was also smallest when the shrinkage
was highest but slightly increased when bloating occurred (Fig. 4(b)). Flexural
strength for the IT0 sample was greatest after firing at 1,250 oC.
Improvement of strength was found in the IT3 sample with the reduction
of optimum temperature to 1,200 oC. Deterioration of the
strength was observed in the IT5 sample primarily due to bloating (Fig.
4(c)).
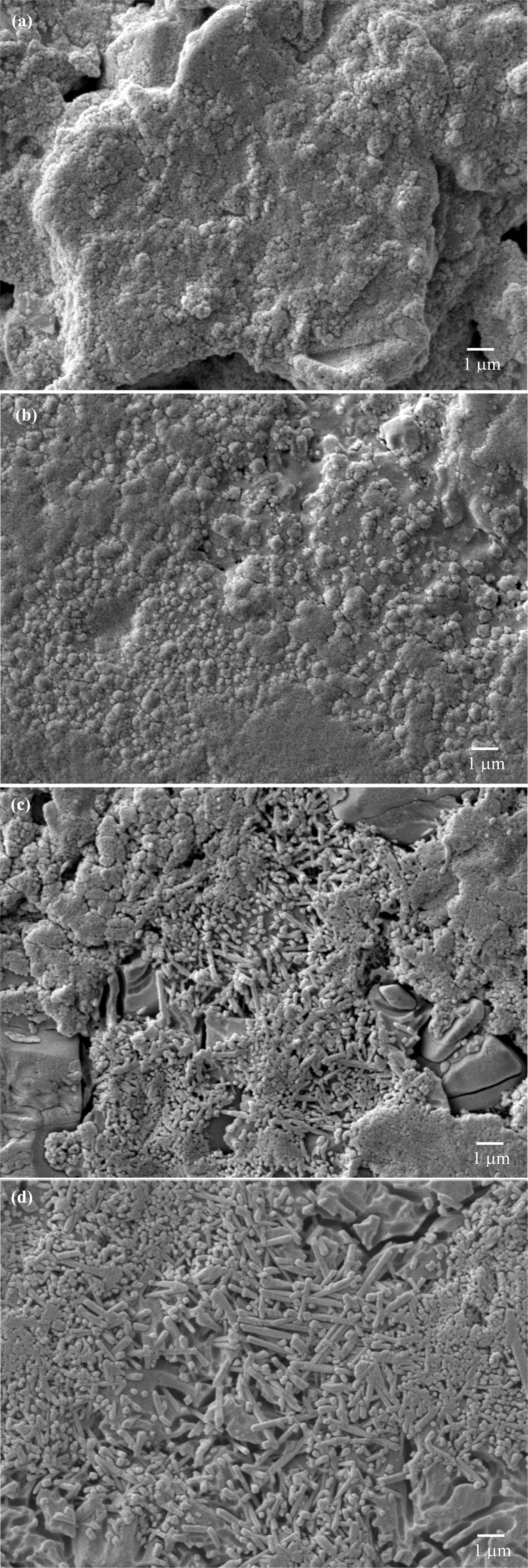
SEM micrographs for the specimens with 3% talc heat treated
at 1,000-1,300 oC are shown in Fig. 5. At 1,000 oC.
The surface was fully covered with nanocrystals and early
stage of densification was also observed (Fig. 5(a)). In the sample with 0%
talc, these crystals were suggested to be mullite formed by
decomposition of illite because primary mullite was not normally observable
at this temperature [20].
Increasing the firing temperature
to 1,100 oC, the nanocrystalline mullite did not appreciably
grow while progressive densification could be observed (Fig. 5(b)). At 1,200 oC, elongated
mullite of up to 1 mm long (as estimated using the SEM scale) appeared while
cubical mullite was still generally observed (Fig. 5(c)). It was noted that
this elongated mullite was laterally larger than needle shaped
mullite typically observed in a standard porcelain body. In a typical porcelain
body where feldspar was used as a single flux, the outer rim of the feldspar
relict showed a clear boundary to the clay relict. Needle shaped-mullite
crystals originated on this boundary and grew into the liquid pool relict. In
this experiment, however, such boundary and liquid pool relict were not so
visible due to the different source and location of the liquid former
being used. Morphology of the secondary mullite was thus
different as a result of the mentioned reason. At 1,300 oC
(Fig. 5(d)), both mullite types grew in size and covered almost all the areas.

|
Fig. 1 SEM micrograph of talc particles revealing their platy shape. |
|
Fig. 2 XRD spectra for talc fired at various temperatures (T: talc, Q: quartz: M: magnesite, D: dolomite). |
|
Fig. 3 XRD spectra for talc fired at various temperatures (T: talc, Q: quartz: M: magnesite, D: dolomite). |
|
Fig. 4 Variation of (a) firing shrinkage (b) water absorption and (c) flexural strength as a function of firing temperatures of the samples with different talc contents. |
|
Fig. 5 Microstructural development of the body with 3% talc addition after firing at (a) 1,000 oC, (b) 1,100 oC, (c) 1,200 oC and (d) 1,300 oC. |
This paper presents the use of talc as in auxiliary flux
to illite in a triaxial body. When it was incorporated in the recipe, phase
transformation up on firing of talc was found to be different from that found
in the pure form. his research showed that talc could be used as a supplemental
flux in the body containing illite as a primary flux
with improved body properties and reduction of the peak
firing temperature. However, the amount of talc contents should not exceed 3
weight % addition otherwise deterioration of the properties especially flexural
strength would be obtained due to bloating of the body.
The authors would like to thank Mae Fah Luang University,
Thailand for the financial and laboratory support.
- 1. A.N.N. Dowuona, A. Yaya, E. Nyankson, J.K. Efavi, L.N.W. Damoah, D. Dodoo-Arhin, V. Apalangya, E. Annan, E.K. Tiburu, B. Onwona-Agyeman, and B. Tomiczek, J. Ceram. Process. Res. 19[2] (2018) 95-100.
- 2. J. Choi, K. Hwang, U. Kim, K. Ryu, K. Shim, and S. Kang, J. Cearmic Process. Res. 20[4] (2019) 424-430.
- 3. R.H. and A.B.A. S. Natrah, Int. J. Autom. Mech. Eng. 16[2] (2019) 6649-6659.
- 4. D.U. Tulyaganov, S. Agathopoulos, H.R. Fernandes, and J.M.F. Ferreira, J. Eur. Ceram. Soc. 26[7] (2006) 1131-1139.
-

- 5. Y.U. and S.Ö.A. Karaa, K. Kayacic, and A.S. Küçükerc, V. Bozkurtd, Ind. Ceram. 29[2] (2009) 71-81.
- 6. L.A. Carús, F. de Souza, and S.R. Bragança, ISRN Ceram. 2012 (2012) 1-7.
-

- 7. S.K. Das and K. Dana, Thermochim. Acta 406[1-2] (2003) 199-206.
-

- 8. K. Dana and S.K. Das, J. Eur. Ceram. Soc. 24[15-16] (2004) 3833-3839.
-

- 9. S.R. Bragança and C.P. Bergmann, J. Eur. Ceram. Soc. 24[8] (2004) 2383-2388.
-

- 10. P. Lima, A. Zocca, W. Acchar, and J. Günster, J. Eur. Ceram. Soc. 38[9] (2018) 3395-3400.
-

- 11. D.Y. Tunçel, M.K. Kara, and E. Özel, IOP Conf. Ser. Mater. Sci. Eng. 18[22] (2011) 222025.
-

- 12. W. Lerdprom, R.K. Chinnam, D.D. Jayaseelan, and W.E. Lee, J. Eur. Ceram. Soc. 36[16] (2016) 4319-4325.
-

- 13. W. Lerdprom, E. Zapata-Solvas, D.D. Jayaseelan, A. Borrell, M.D. Salvador, and W.E. Lee, Ceram. Int. 43[16] (2017) 13765-13771.
-

- 14. D. Wattanasiriwech, K. Srijan, and S. Wattanasiriwech, Appl. Clay Sci. 43[1] (2009) 57-62.
-

- 15. A. Aras, Appl. Clay Sci. 24[3-4] (2004) 257-269.
-

- 16. R. E. Grim and W. F. Bradley, J. Am. Ceram. Soc. 23[8] (1940) 242-248.
-

- 17. A. Khalfaoui, S. Kacim, and M. Hajjaji, J. Eur. Ceram. Soc. 26[1-2] (2006) 161-167.
-

- 18. G. Wang, H. Wang, and N. Zhang, Appl. Clay Sci. 146 (2017) 254-263.
-

- 19. S. Boussen, D. Sghaier, F. Chaabani, B. Jamoussi, and A. Bennour, Appl. Clay Sci. 123 (2016) 210-221.
-

- 20. D. Wattanasiriwech and S. Wattanasiriwech, J. Eur. Ceram. Soc. 31[8] (2011) 1371-1376.
-

- 21. S. Farrokhpay, B. Ndlovu, and D. Bradshaw, Appl. Clay Sci. 160 (2018) 270-275.
-

- 22. A. Dumas, F. Martin, E. Ferrage, P. Micoud, C. Le Roux, and S. Petit, Appl. Clay Sci. 85 (2013) 8-18.
-

- 23. M. Hojamberdiev, P. Arifov, K. Tadjiev, and Y. Xu, Min. Sci. Technol. 20[3] (2010) 415-420.
-

- 24. L. Esposito, A. Salem, A. Tucci, A. Gualtieri, and S.H. Jazayeri, Ceram. Int. 31[2] (2005) 233-240.
-

- 25. M.U. Taskiran, N. Demirkol, and A. Capoglu, J. Eur. Ceram. Soc. 25[4] (2005) 293-300.
-

- 26. M. Dondi, V. Biasini, G. Guarini, M. Raimondo, A. Argnani, and S. Di Primio, Key Eng. Mater. 206-213[II] (2001) 1795-1798.
-

- 27. L.A. Pérez-Maqueda, A. Duran, and J.L. Pérez-Rodríguez, Appl. Clay Sci. 28[1-4] (2005) 245-255.
-

- 28. F.O. Aramide, O.D. Adepoju, and A.P. Popoola, J. Ceram. Process. Res. 19[6] (2018) 483-491.
- 29. C.J. Ngally Sabouang, J.A. Mbey, F. Hatert, and D. Njopwouo, J. Asian Ceram. Soc. 3[3] (2015) 360-367.
-

- 30. T. Iiyama, Proc. Jpn. Acad. 31[3] (1955) 166-168.
-

- 31. N.N. Sampathkumar, A.M. Umarji, and B.K. Chandrasekhar, Mater. Res. Bull. 30[9] (1995) 1107-1114.
-

- 32. F.A.C. Oliveira, N. Shohoji, J.C. Fernandes, and L.G. Rosa, Sol. Energy 78[3] (2005) 351-361.
-

- 33. S.L. Correia, K.A.S. Curto, D. Hotza, and A.M. Segadães, Mater. Sci. Forum 498-499 (2009) 447-452.
-

- 34. D. Wattanasiriwech, C. Sangtong, and S. Wattanasiriwech, ScienceAsia 33[1] (2007) 125-130.
- 35. S.Y.R. López and J.S. Rodríguez, AZoJomo, 4[July] (2008) 1-7.
 This Article
This Article
-
2019; 20(6): 643-648
Published on Dec 31, 2019
- 10.36410/jcpr.2019.20.6.643
- Received on Jul 19, 2019
- Revised on Oct 8, 2019
- Accepted on Oct 20, 2019
 Services
Services
- Abstract
introduction
experiments
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Darunee Wattanasiriwech
-
aCenter of Innovative Materials for Sustainability, School of Science, Mae Fah Luang University, Thailand
bCircular Economy for Waste-free Thailand Research Group, Mae Fah Luang University, Thailand
Tel : +6653916263 Fax: +6653916776 - E-mail: darunee@mfu.ac.th






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.