- Effect of B2O3 addition on the Sintering of BaTiO3-CaTiO3 Composite Materials
T. Bezzia,*, A. Chennia, F. Sellama, K. Sahraouib and S.E. Baramab
aNational Center For Biotechnology Research, Ali Mejli Nouvelle Ville, UV03 Bp E73, Constantine, Algeria
bCeramics Laboratory, Fréres Mentouri University of Constantine 1, Algeria
The effects of B2O3
addition on the sintering behaviour of BaTiO3-CaTiO3 (BCT)
materials were investigated. (1-x) BaTiO3-x CaTiO3-y B2O3
(where x is 3 or 7 % mol. and 0 ≤ y ≤ 3 mol.% respectively). Pellets were
prepared from BaTiO3 (BT) and CaTiO3 (CT) powders mixture
and sintered at 1,200, 1,250, 1,300 and 1,350 oC, gradually in
air for 3 h. The relative density of BCT samples with 1 mol. % B2O3
sintered at 1,200 oC were as good as those of undoped BCT
sintered at 1,350 oC. When > 2 mol. % B2O3
was added to BCT, the over doped B2O3 did not form a
liquid phase or volatilize, it remained in the samples and formed a secondary
phase that lowered the sintering behaviour of the BCT. The X-Ray diffraction
analysis carried out on both kind of pellets (x = 0.3 and
x = 0.7) revealed the presence of BT and CT solutions together in an
unidentified secondary phase.
Keywords: BaTiO3, CaTiO3, B2O3, Sintering, Relative density, X-Rays diffraction
The nonlinear properties of ferroelectrics materials have,
recently, became the subject of intense research activity both in the
fundamental and practical domains. These materials, are currently used in
manufacturing of various microwave devices, such as voltage-controlled
oscillators (VCO), tenable filters, phase shifters and varactors
[1-3]. For such application, the basic requirements are;
high dielectric tenability defined as the mean variation
of the relative permittivity with field intensity, low dielectric
loss tan δ, appropriate level of dielectric constant and a feeble
temperature dependence of permittivity. In general, Strontium Titanate (ST)
based materials are used for devices operation at low temperature while (Ba,
Sr) TiO3 (BST) based materials are used for devices operating
at room temperature [4, 5]. Ferroelectric based materials
present two major inconvenient, relatively high dielectric loss and larger
temperature dependence of their relative permittivity. An efficient way is to
dope oxides that have low dielectric loss into the ferroelectric materials. Additionally,
the introduction of oxides would also be able to modulate the dielectric
properties of these materials for specific applications. CaTiO3
is a typical depressor in BaTiO3 ceramics, which
conduces to a significant decrease both in dielectric
loss and in temperature coefficient of dielectric constant.
Furthermore, CaTiO3 introduces only slight change of Curie point
when Ca2+ substitute Ba2+ in the A sites [6] but
causes an important shift to lower temperature when Ca2+
substitute Ti4+ in B sites [7]. In this latter case, it would be
possible to obtain like diffuse phase structure conferring good dielectric and
relaxer properties to these materials. Consequently, (Ba, Ca) TiO3
systems are expected to be alternative candidates for tunable microwave dielectric
materials with low dielectric loss and small temperature dependence [8].
BT or BCT ceramics usually
require sintering tempera- ture of
> 1,300 oC. Such temperatures are, unfortunately, too high for industrial
manufacturing. The principal objective of the present investigation was to
decrease the sintering temperature of BCT based materials by introducing B2O3
with different proportion in the initial powder mixture. B2O3
is well known as a compound with low melting point, that would react with CaO and
BaO to form glass phase, which
enhance the liquid sintering process and consequently increase the densification
of the sintered materials.
The BaTiO3 (BT) powder used in this
investigation was reagent commercial type manufactured by FLUKA while the used
CaTiO3 (CT) powder was prepared in laboratory following the
conventional procedure of milling and calcination. The starting raw materials
used for CT preparation were high purity commercial anatase
TiO2 powder (also made by FLUKA) and local CaCO3 powder
(extracted out from Khroub locality in Algeria). The
starting powders were weighed and mixed in a stoichiometric proportion. The
mixtures were then ball-milled for 6h using alumina balls in methanol alcohol.
The obtained slurry was dried at 70 oC in electrical furnace
and calcined in air at 1,100 oC for 8 h. XRD analysis was
carried out on calcined powders to control the formation of CT phase. (1-x)
BaTiO3-x CaTiO3-y B2O3 (where x is
3 or 7% mol. respectively and 0 ≤ y ≤ 3 mol.%) mixtures pellets were prepared
from BaTiO3 (BT), CaTiO3 (CT) and B2O3 powders
and re-milled by the same route. 1 mm high and 13 mm diameter pellets were
obtained by uniaxial cold pressing under 70 MPa. The pellets were then sintered
at 1,200-1,350 oC
temperature range. The sintered samples were characterised in terms of apparent
density by direct measure of mass and
volume. X-Ray diffraction analysis
was carried out on different sintered samples to follow the microstructure
evolution.
Study
of sample containing 70% of BT
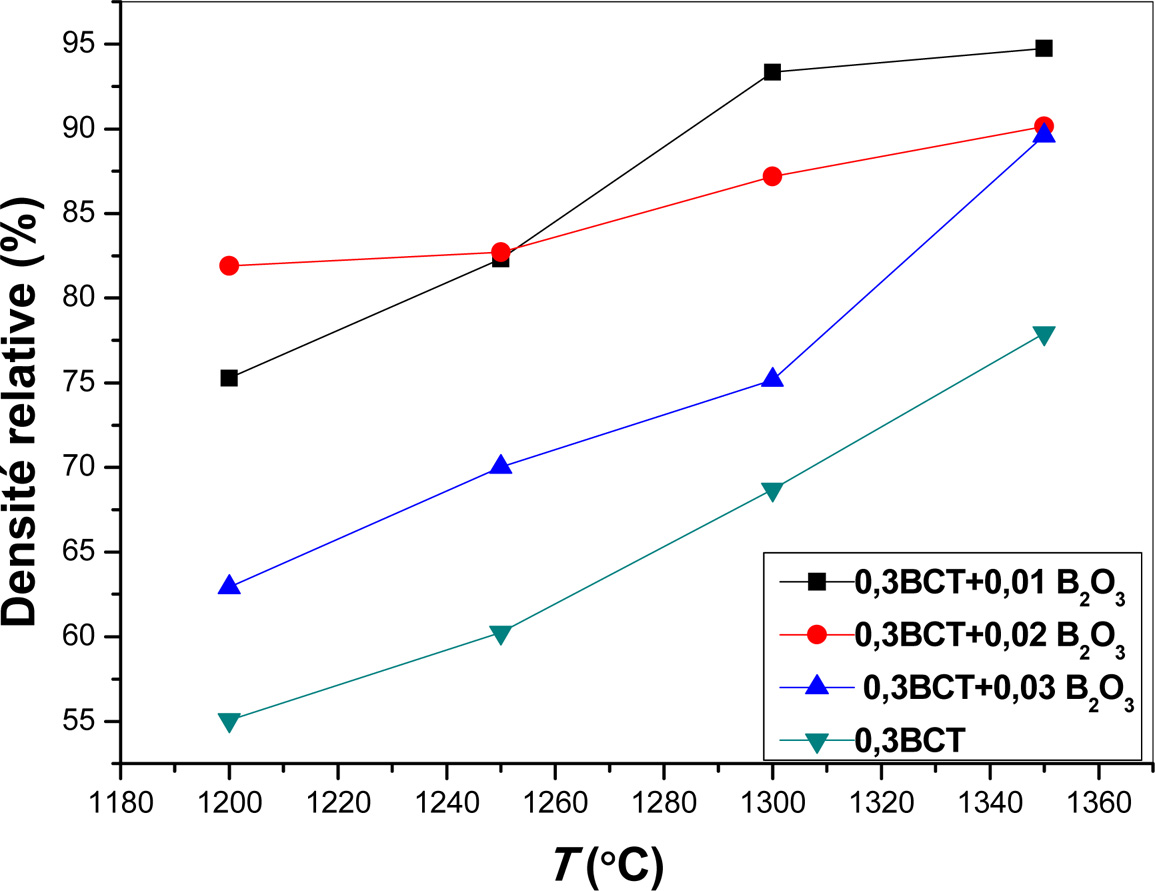
The relative densities of samples sintered for 3 h at
different temperatures are reported in Fig. 1. The obtained
curves reveal identical evolution of the relative density as a
function of the sintering temperature for all considered B2O3 proportion
in the initial mixture. We noticed as well that the increase in the relative
density is, in all cases, about 17 to 20% per 150 oC. This
result, probably, reveals identical densification mechanisms of pellets in this
range of temperature.
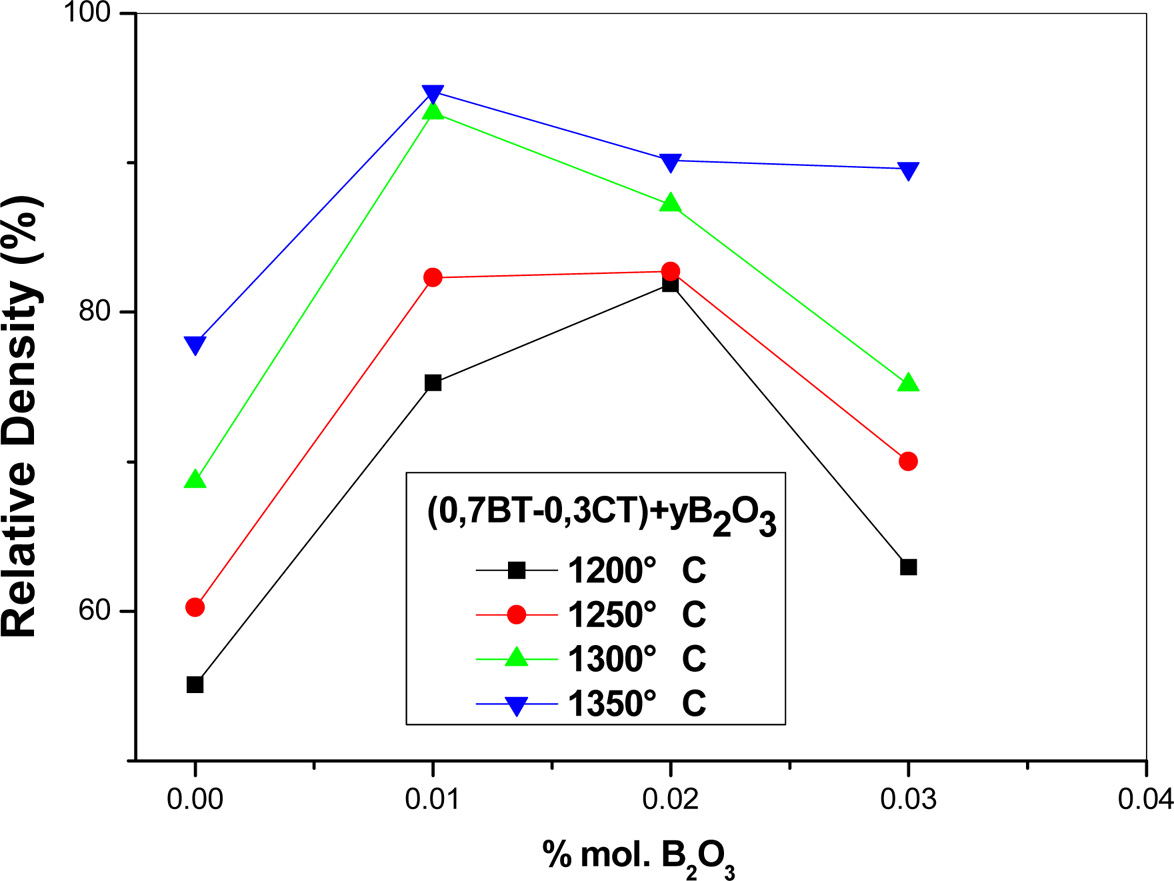
On the other hand, the obtained findings show (Fig. 2) an important
increase in the relative density as a function of B2O3
additions up to 1% mol. At the opposite, the
relative density of pellets decreases beyond such a percentage. Moreover, It can be noticed that the decrease in the
relative density is the more important that the sintering temperature is lower.
This evolution is probably due to the formation of liquid phase, which enhances
the densification for moderate proportion of B2O3 by
developing liquid sintering process. When the proportion of liquid phase became
so important (for Higher B2O3 proportion), the apparition
of secondary porosity (Fig. 2) inhibits the sintering, consequently the pellet
density decreases.
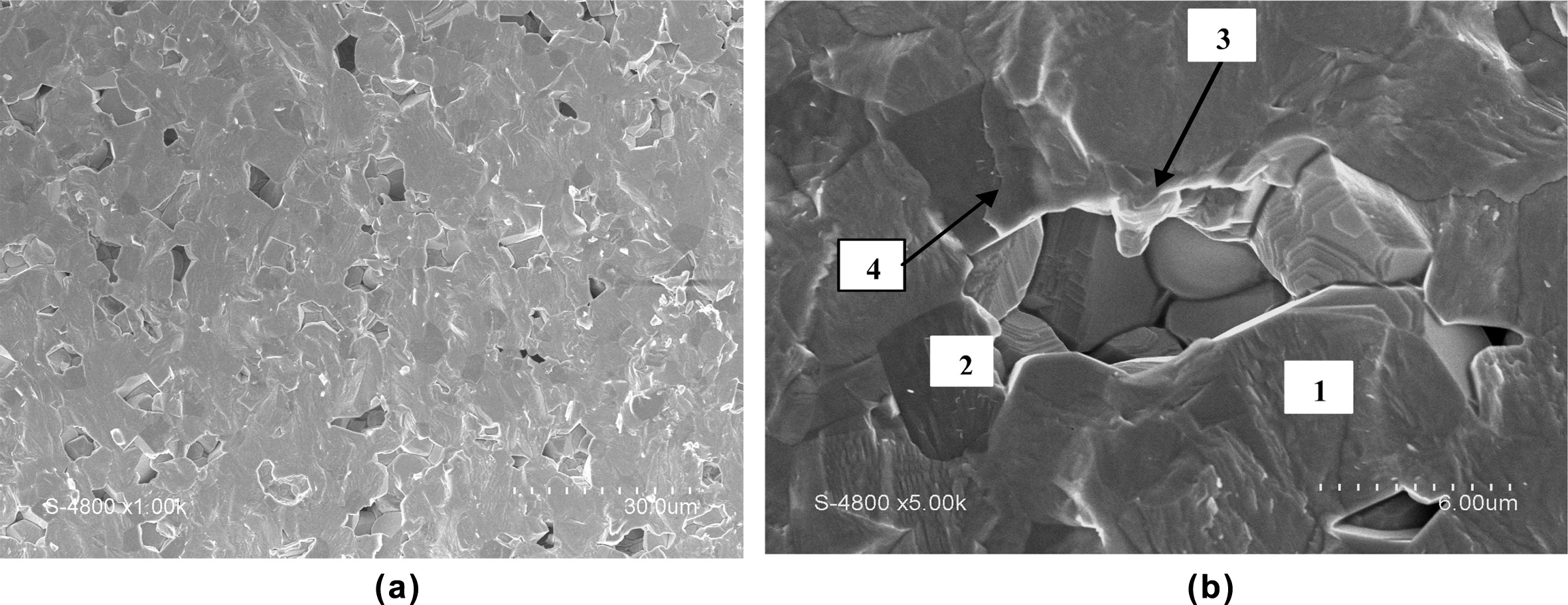
Higher B2O3 proportion), the
apparition of secondary porosity (Fig. 3(a)) inhibits the sintering,
consequently the pellet density decreases.
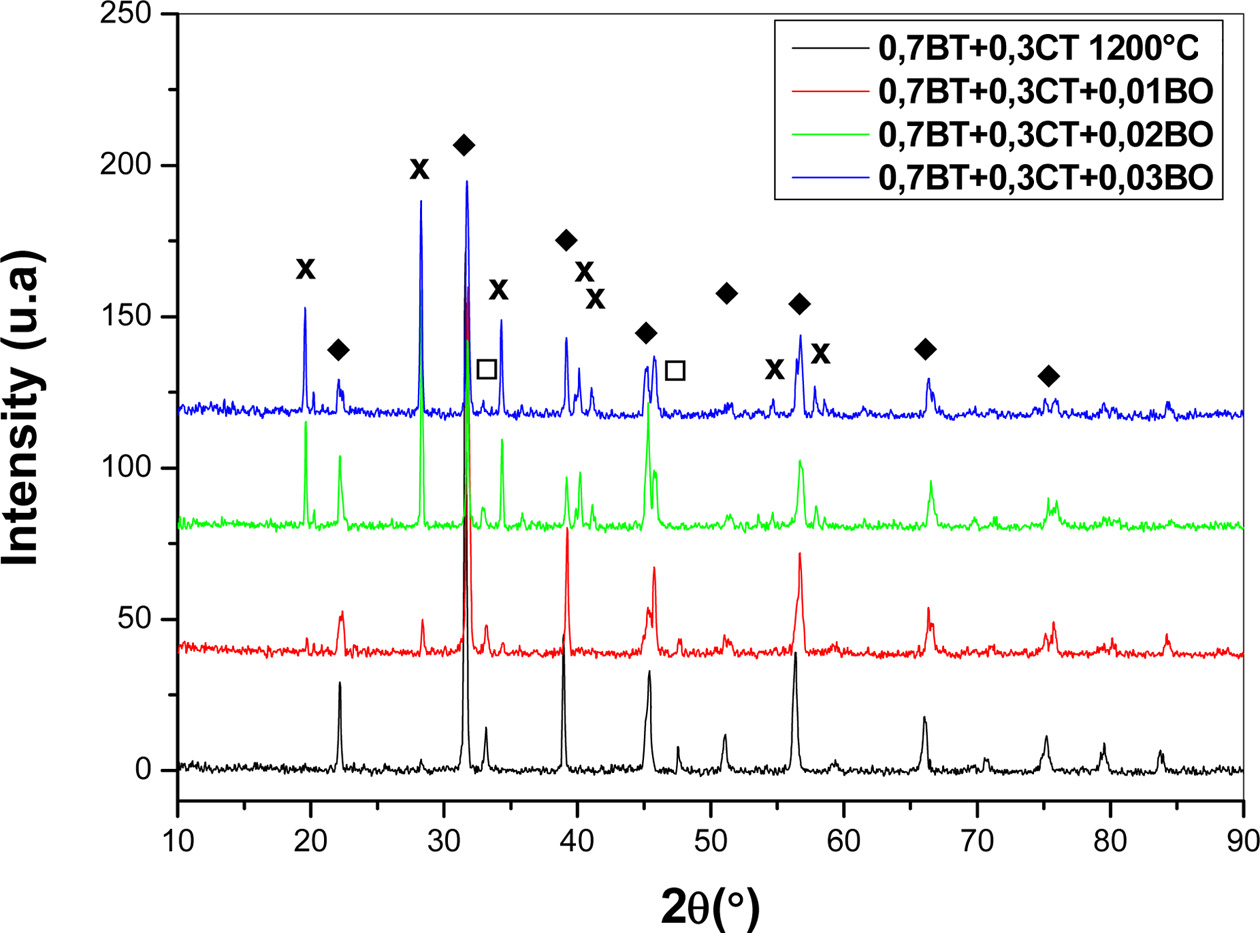
The formation of liquid phase is probably the con- sequence of the reaction between BT, CT
and B2O3 at lower temperature. X-Ray diffraction spectra
recorded from different pellets effectively put in evidence the formation of BT and CT
solution together an un-identified secondary
phase for all considered sintering temperatures (Fig. 4). Furthermore, the
obtained results reveal a decrease in
proportions of BT and CT solutions
respectively as function of added B2O3, while, the
pro- portion of the unidentified phase increases. Consequently, the unidentified phase probably corresponds to
liquid phase formed before than 1,200 oC.
Fig. 3(b) reveals the presence of 3 phases; the BT solid
solution major phase (1), a minor dark phase (2) corresponding
to CT solid solution and a glass-ceramics phase (3)
designated by an arrow. Furthermore, it can be seen that the melting phase
wraps a great area of a CT grain in zone (4)
and is neighboured by secondary porosity
which probably appears because of the shrinkage of the liquid phase during pellet cooling. Hence it must be concluded
that the formation of secondary liquid phase
enhances the densification of pellets for moderate proportion
of B2O3 by developing liquid sintering process. Contrariwise, when liquid phase content
increases, the proportion of the
secondary porosity, induced by the liquid phase shrinkage, increases too and
leads to a diminution of pellets density.
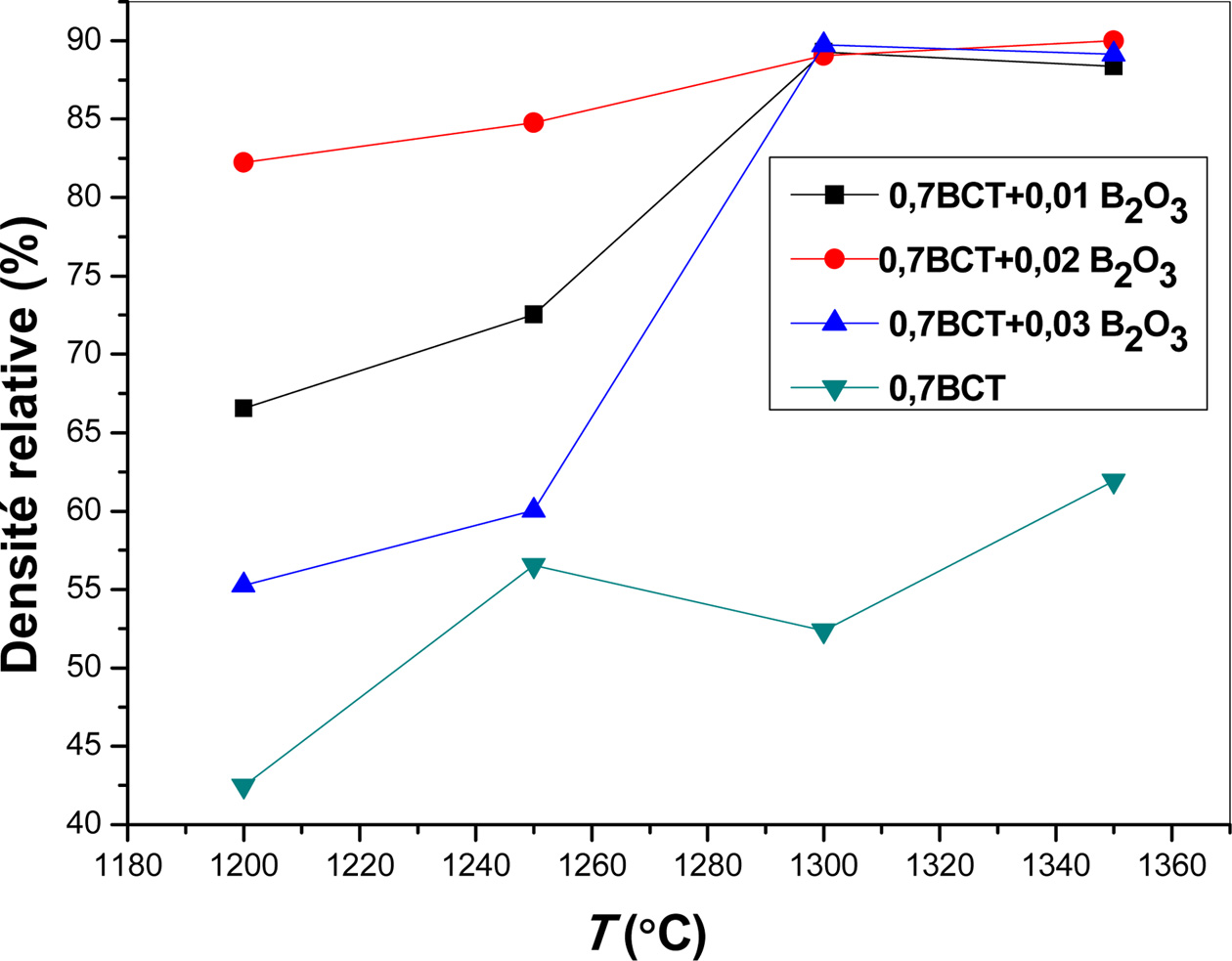
Study
of sample containing 70% of CT
The relative densities of samples containing 70% mol. of
CT and sintered during 3 h at different temperatures
are reported in Fig. 5.
As it can be observed, the evolution of the relative
density is quiet different from that obtained in the case of sample containing
30% mol. of CT. Hence, the relative density increases between 1,200 and
1,300 oC and reaches a maximum of about 90% at 1,350 oC
for all considered B2O3 percentages. At the same time,
the precedent curves reveal an important effect of B2O3
addition on the relative density. This fact is more apparent as we report the
evolution of the relative density as a function of added B2O3
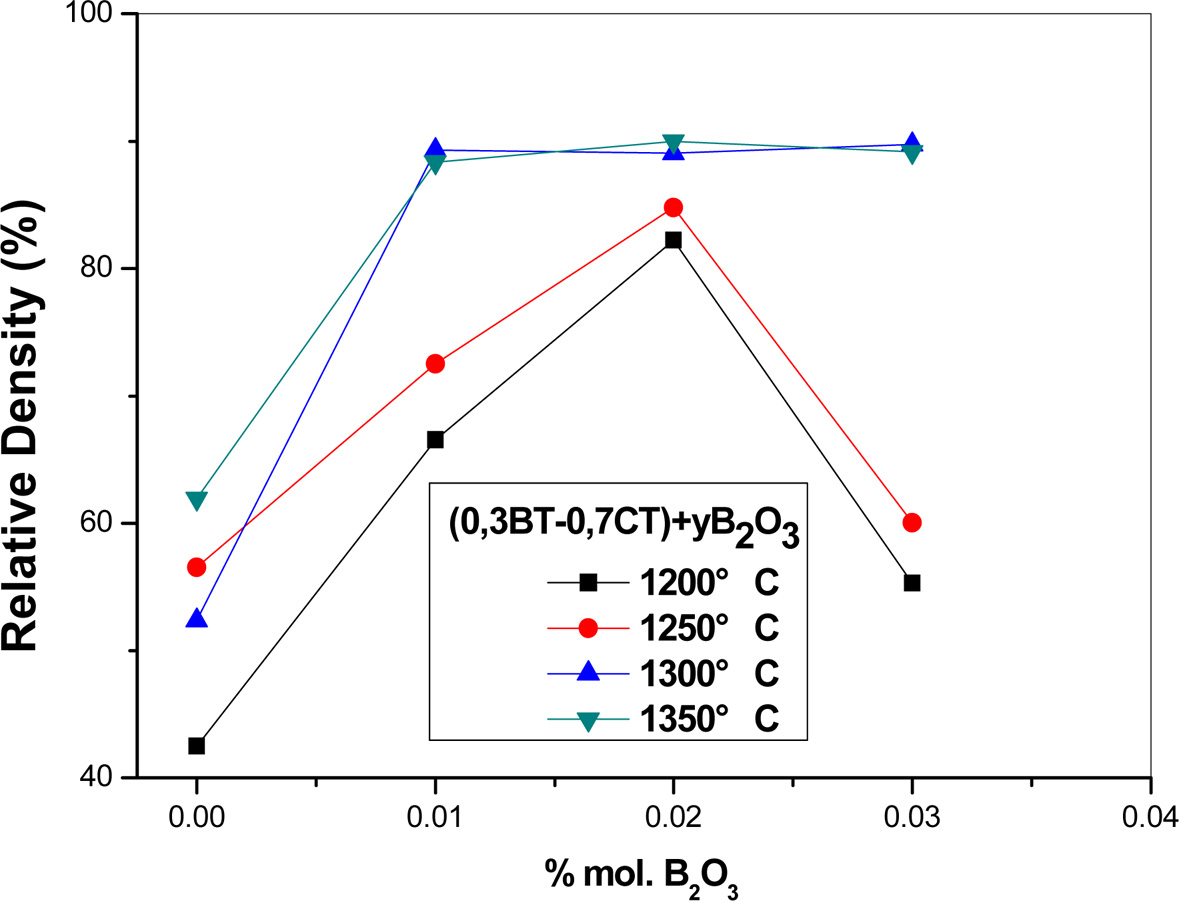
percentage. In the case of pellets sintered at 1,200 or 1,250 oC,
the obtained curves (Fig. 6) firstly reveal an important
increase in the relative density with added percentage of B2O3 up
to 2% mol. For example, the relative density in pellets sintered
at 1,200 oC pass from 42.3% in undoped sample
to 82.3% in sample containing 2 mol.% of B2O3.
This corresponds to an augmentation of 100 per 100 in the
relative density. Over 2% mol of added B2O3, the relative
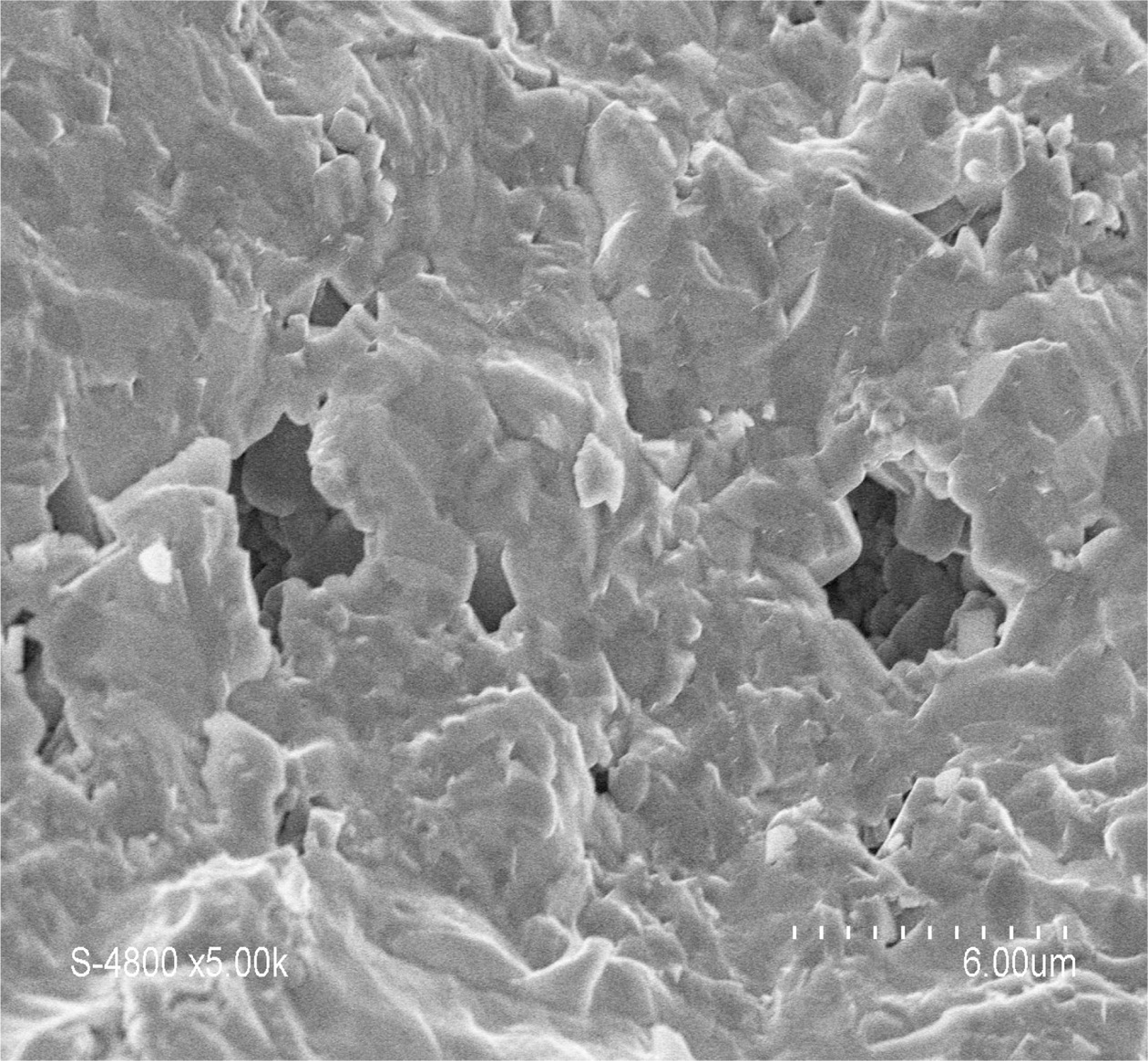
density decreases again probably owing to the formation of an important liquid
phase proportion leading to the apparition of secondary porosity (Fig. 7) which
restrain the densification in this range of temperature.
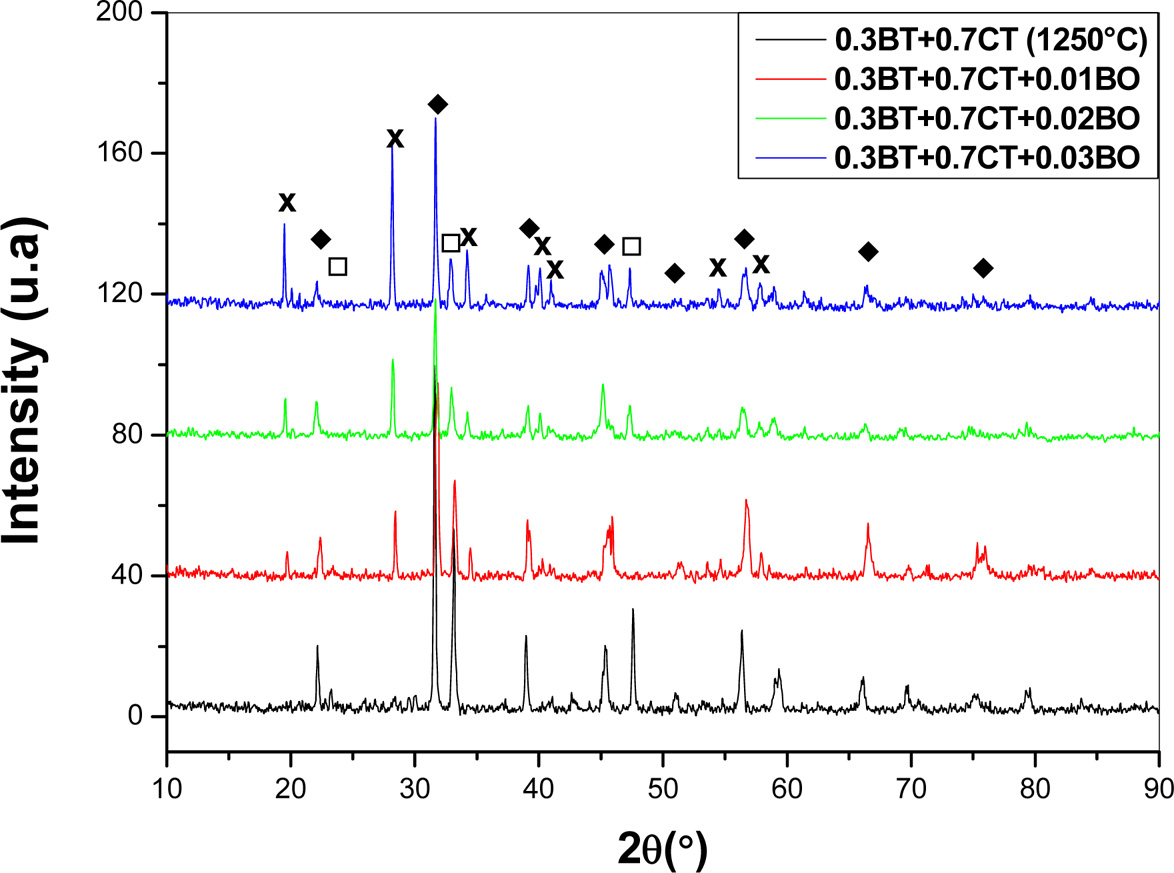
As in the case of pellets containing 30% of CT, the liquid
phase probably corresponds to the unidentified phase revealed by X-Ray
diffraction spectra recorded from different samples (Fig. 8). The spectra put
in evidence the presence of BT solid solution, CT solid solution and
unidentified phase whose proportion increases with added percentage of B2O3.
Nonetheless, it is important to remark that the pro- portion of BT solid solution seems not be
affected by B2O3 addition, while, CT
proportion manifestly diminishes as mol.% of B2O3
is increased. Therefore, we can say that the unidentified phase
principally forms by reaction between CT and B2O3.
In the case of pellets sintered at 1,300 or 1,350 oC,
the corresponding curves reveal, at the beginning, an increase in the relative
density with added B2O3 then the relative density
achieves a maximum of about 90% as B2O3 addition exceeds
1% mol.
|
Fig. 1 Relative density of different ((1 - y)(0.7 BaTiO3 + 0.3 CaTiO3) + yB2O3 pellets as function of sintering temperature. |
|
Fig. 2 Relative density of different ((1 - y)(0.7 BaTiO3 + 0.3 CaTiO3) + yB2O3 pellets as function of proportion of added B2O3. |
|
Fig. 3 (a) MEB micrograph of BT-0.3 CT + 3% B2O3 pellet sintered at 1250 oC for 3 h and (b) SEM micrograph of 0.7BT-0.3 CT + 3% B2O3 pellet sintered at 1,250 oC for 3 h. |
|
Fig. 4 X-Ray diffraction spectra recorded from different ((1 - y) (0.7 BaTiO3 + 0.3 CaTiO3) + yB2O3 pellets sintered at 1,200 oC from 3 h; BT, CT, X unidentified phase. |
|
Fig. 5 Relative density of different ((1 - y) (0.3 BaTiO3 + 0.7 CaTiO3) + yB2O3 pellets as function of sintering temperature. |
|
Fig. 6 Relative density of different ((1 - y)(0.3 BaTiO3 + 0.7 CaTiO3) + yB2O3 pellets as function of proportion of added B2O3. |
|
Fig. 7 MEB micrograph of BT-0.7 CT + 3% B2O3 pellet sintered at 1,200 oC for 3 h. |
|
Fig. 8 X-Ray diffraction spectra recorded from different ((1 - y) (0.3 BaTiO3 + 0.7 CaTiO3) + yB2O3 pellets sintered at 1,250 oC from 3 h; BT, CT, X unidentified phase. |
The relative density of (1-x) BaTiO3-x CaTiO3-y
B2O3 (where x is 3 or 7% mol.
respectively and 0 ≤ y ≤ 3 mol.%) composite materials depends on both chemical
composition of the initial mixtures and sintering conditions.
The reaction between BT, CT and BO powder conduces
to the formation of liquid phase which enhances the densification
of pellets, since its proportion is moderate. Inversely,
the liquid phase inhibits the densification when its proportion became so
important.
In the case of pellets containing 30% of CT, the obtained
results reveal an increase in the relative density of about 20% corresponding
to an increase of 150 oC in the sintering temperature. A
maximum of densification (~95%) is obtained in pellet containing 1% mol. of B2O3
and sintered at 1,300 oC.
The addition of 1% mol. of B2O3
provokes an increase of about 20% in the relative density for all considered
sintering temperature. At the opposite, the relative density decreases for
higher percentage of B2O3 addition.
In the case of pellets containing 70% of CT, the obtained
results also reveal an increase in the relative density with sintering
temperature of about 20% corresponding to an increase of 150 oC.
Like in the case of pellets containing 30% CT, the addition
of B2O3, up to 2% mol, enhance the densification,
but the relative density decreases over this percentage in the case of pellets
sintered at 1,200 or 1,250 oC. For higher
sintering temperature, the relative density reaches a maximum of
about 90% as B2O3 addition exceeds 1% mol.
- 1. A.B. Kozyrev, T.B. Samoilova, A.A. Golovkov, E.K. Hollmann, D.A. Kalinikos, V.E. Loginov, A.M. Prudan, O.I. Soldatenkov, D. Galt, C.H. Mueller, T.V. Rivkin, and G.A. Koepf, J. Appl. Phys. 84[6] (1998) 3326-3332.
-

- 2. C.L. Chen, H.H. Feng, Z. Zhang, A. Brazdeikis, Z.J. Huang, W.K. Chu, C.W. Chu, F.A. Miranda, F.W. Van Keuls, R.R. Romanofsky, and Y. Liou, Appl. Phys. Lett. 75[3] (1999) 412-414.
-

- 3. X.X. Xi, H.C. Li, W.D. Si, A.A. Sirenko, I.A. Akimov, J.R. Fox, A.M. Clark, and J.H. Hao, J., Electroceram. 4[2/3] (2000) 393-405.
- 4. L.B. Kong, S. Li, T.S. Zhang, J.W. Zhai, F.Y.C. Boey, and J. Ma, Progress in Materials Science 55[8] (2010) 840-893.
- 5. W.T. Chang and L. Sengupta, J. Appl. Phys. 92[7] (2002) 3941-3946.
- 6. M.S. Yoon and S.C. Ur, Ceram. Int. 34[8] (2008) 1941-1948.
- 7. J.G. Park, T.S. Oh, and Y.H. Kim, J Mater Sci. 27[11] (1992) 5713–5719.
-

- 8. X.M. Chen, T. Wang, and J. Li, Mat, Sci. and Eng. B 113[2] (2004) 117-120.
- 9. R.C. Devries and R. Roy, J. Am. Ceram. Soc. 38[4] (1955) 142-146.
-

 This Article
This Article
-
2019; 20(6): 617-620
Published on Dec 31, 2019
- 10.36410/jcpr.2019.20.6.617
- Received on May 31, 2019
- Revised on Oct 8, 2019
- Accepted on Oct 20, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- T. Bezzi
-
National Center For Biotechnology Research, Ali Mejli Nouvelle Ville, UV03 Bp E73, Constantine, Algeria
Tel : +213 (31) 77 50 37/39 Fax: +213 (31) 77 50 44 - E-mail: t.bezzi@crbt.dz






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.