- A study on the ball-milling effect of calcined Mg-Al hydrotalcite on the CO2 adsorption behavior
F. Granados-Correa*, J.L. Iturbe-García and J. Bonifacio-Martínez
Instituto Nacional de Investigaciones Nucleares, Departamento de Química, A.P. 18-1027, Col. Escandón, Delegación Miguel Hidalgo, C.P. 11801, Ciudad de México, México
We have investigated the
effect of ball-milling over calcined Mg-Al hydrotalcite, with regard to its CO2
adsorption capacity. The prepared materials were systematically characterized
by X-ray diffraction, scanning electron microscopy and N2
physisorption measurements. The results reveal an excellent adsorption capacity
of CO2 by calcined Mg-Al hydrotalcite; however, when this adsorbent
was treated by ball-milling for 2 h, a slight decrease in its CO2
adsorption capacity was observed, mainly attributable to the decreased surface
area, total pore volume, pore size and the formation of particle aggregates of
the aforementioned hydrotalcite. Therefore, alterations in structure, texture
and morphology of calcined Mg-Al hydrotalcite during the ball-milling treatment
do not optimize its CO2 adsorption capacity.
Keywords: CO2 adsorption, Calcined Mg-Al hydrotalcite, Co-precipitation synthesis, Ball-milling effect.
It is well-known that global warming is one of the main
environmental concerns around worldwide, and that CO2 is one of the
greenhouse gases that most notably contribute to this problem [1]. Currently,
it is an environmental issue of great importance that requires an immediate
solution to diminish the negative impacts of high CO2 atmospheric
concentrations to the environment and ecosystems [2]. In this framework, the
global scientific community has made great efforts to develop
new and advanced CO2-capture technologies as
technologically viable solutions for the reduction of atmospheric CO2
on a large scale. In this scenario, recently, one of the most promising
post-combustion CO2 capture technologies has been developed, which
carries out CO2 capture with solid adsorbents, made possible by its
high operating flexibility and low energy demand; thus, from this point of
view, the design of CO2 solid adsorbents is quite viable
[3, 4]. Many solid adsorbents have been actively researched and reported
in specialized literature for CO2 capture purposes [5-10]. Each one
of these studied materials has shown interesting results in
terms of CO2 adsorption capacity, under different
temperature and pressure conditions, but it is still necessary to find an
adsorbent that exhibits high selectivity and adsorption towards CO2;
besides, great effort has been made to improve CO2 capture. In
this regard, the key requirement for technological CO2
capture applications is the development of new
adsorbents that have a high CO2 working retention
capacity over a wide temperature range, from room temperature to 700 oC
[11]. Currently, and according to literature, hydrotalcites-type adsorbents,
with divalent and trivalent cations such as Mg2+ and Al3+,
have demonstrated exceptional CO2 capture due to their unique
characteristics, such as anion-exchange, high surface area
and a considerable layer-charge, significant with regard
to acidic gases, as in the case of CO2 [12-14]. Hydrotalcites can be
obtained commercially, as well as in a laboratory; they are
double-layered hydroxides with lamellar structure, formed by a
positively-charged brucite layer, with an interlayer space to accommodate water
molecules and CO32- anions. Also,
these materials have presented high stability during cycling operations,
which is crucial for the development of practical applications such as CO2
capture [15, 16]. It has been reported in the literature,
that the maximum CO2 capture capacity of
hydrotalcite compounds are generally found in the temperature range of
200-400 oC [17-19]. On the other hand,
it has also been confirmed that the adsorbent surface area
plays an important active role in CO2 adsorption, because it
translates to a greater number of active sites which, in turn,
represent greater adsorbance. To address this issue, different
strategies have been examined in recent years in order to improve adsorbent
activity [20-23]. In particular, the strategies that include reducing the
adsorbent particle-size to nanometric scale (<100 nm) have proved to be promising
methodologies for improving adsorbent activity, because the generated fine
nanoparticles have higher contact and reaction efficiencies than traditional
materials do [24]. It is well-known that the ball-milling mechanical activation
method provides an efficient route for preparing stabilized
nano-size adsorbents with improved structural, chemical
and textural characteristics that could be relevant for CO2 capture
[25]. The mechanical ball-milling method is an extensive method employed to obtain
nanostructured materials with several technological uses
[26-27]; this mechanical ball-milling treatment allows to activate dry solids
and specifically increase their surface area, as well as to improve their CO2
capture
properties [28]. Thus, it is considered to have a broader impact compared to
other conventional materials. With regards
to hydrotalcite-like compounds, the
recent interest in novel mechanochemical approaches is
devoted almost exclusively to the successful preparation and modification of these layered double
hydroxides using different starting materials, as well as preparing different nanocomposites [29-32]. From the
hydrotalcite synthesis viewpoint, the
mechanochemical activation method is
simple and versatile, reduces time consuming, and avoids heating
treatment, solvent use and production
of large amounts of waste, which are characteristics consequences of other
existing synthesis methods such as urea hydrolysis, sol-gel, hydrothermal,
combustion, sonication, microwave irradiation, steam activation and
solvothermal [33]. So far, however, significantly fewer studies have indicated
that the ball-milling process, as a nanoparticle generation method, enhances CO2
capture [11, 25].
With regard to this concern,
studies need to be conducted in order to determine the effect of ball milling over the CO2
adsorption behavior of hydrotalcites in order
to optimize this process. To the best of our knowledge, no study has been reported on CO2 adsorption behavior of calcined Mg-Al hydrotalcite after ball-milling. Therefore, new
results could contribute to a greater understanding of the
CO2 adsorption mechanism. For these reasons, the aim
of this study was to understand the effectiveness of the calcined Mg-Al
hydrotalcite, followed by ball-milling treatment for CO2 capture, as
a viable CO2 capture process, because of the expected formation of
aluminum and manganese oxide coatings over the Mg-Al calcined hydrotalcite
surface, which may provide different adsorption sites and a higher surface area
for enhanced CO2 adsorption due to its resultant nanostructured
nature.
The Mg-Al hydrotalcite used in this study was synthetized
by the co-precipitation method at constant pH, according to Sato et al.
[34]. This procedure is based on the mixing of solutions A and B;
solution A contains the cation precursors of hydrotalcite (Mg2+
and Al3+) in water and solution B consists of NaOH and NaCO3
compounds, which provides a basic medium of pH between 8-10, sufficient for
hydrotalcite formation and precipitation. These carbonates
participate in the stabilization of hydrotalcite structure as load
compensators, due to its large size, which facilitates the filtration
of the precipitate formed. Then, using a classic procedure,
1,000 mL of a solution of MgCl2·6H2O 0.75 M and AlCl3·6H2O
0.25 M were added, drop by drop, to 1000 mL of another solution, which
consisted of 0.5 M Na2CO3 and 2.5 M NaOH, under vigorous
agitation. Both aqueous solutions were previously heated to 60 oC
and kept at this temperature during vigorous agitation. Once the
hydrotalcite was obtained, it was separated by filtration and then washed with
enough distilled water until that the chloride ions were not detectable by
precipitation with silver nitrate in solution. The Mg-Al hydrotalcite
precipitate was dried at room temperature for 5 days, and then dried at
80 oC in a stove for three hours. The molar ratio value of
prepared hydrotalcite by this method was 0.25. Then, the calcined Mg-Al
hydrotalcite, denoted as CHT, was obtained by heating the previously-prepared
Mg-Al hydrotalcite to 500 oC for 5 hours in a muffle furnace;
the obtained powders were cooled to room temperature and grounded to a fine
powder in an agate mortar. Additionally, the CHT prepared via co-precipitation,
followed by calcination, was ball-milled for 2 hours under an argon atmosphere
by using a Spex 8000 high-energy mechanical mill with a 50 mL-capacity and a
ball-to-powder weight ratio of 6:1. This obtained material
(CHT-BM), together with the dry Mg-Al calcined hidrotalcite (CHT),
were employed for CO2 adsorption determinations.
CO2 adsorption experiments were carried out at
200 oC and 1 atmosphere of pressure using a Parr 4592
stainless-steel pressure reactor with a 50 mL-capacity, coupled to a
temperature-controlled system. Separately, 8 mg of Mg-Al hydrotalcite samples
were exposed to a high-purity (99.98%), ultra-dry CO2 gas flow for
15 minutes, as a fixed saturation time. Before the CO2 adsorption
tests, the samples were pretreated at 325°C under vacuum for 30 min in order
to remove the adsorbed environmental
impurities. CO2 adsorption capacities
were determined by thermogravimetric analysis using a TGA calorimeter analyzer (TA Instruments SDT Q600), coupled to
a mass spectrum analyzer (TA Instruments, LLC). In this analysis, approximately
6 mg of Mg-Al hydrotalcite samples were placed in a ceramic cell and heated
from 20°C to 850°C at a heating rate of 10 °C/min, under an inert atmosphere,
in which helium was used as the carrier gas (100 mL/min). The ratio m/e = 44 was used for CO2
quantification. The number of
millimoles of CO2 captured per gram of Mg-Al hydrotalcite (mmol/g) were
calculated from the TGA calcination profiles, based on the weight loss of CO2
that was adsorbed in the materials.
XRD
analysis
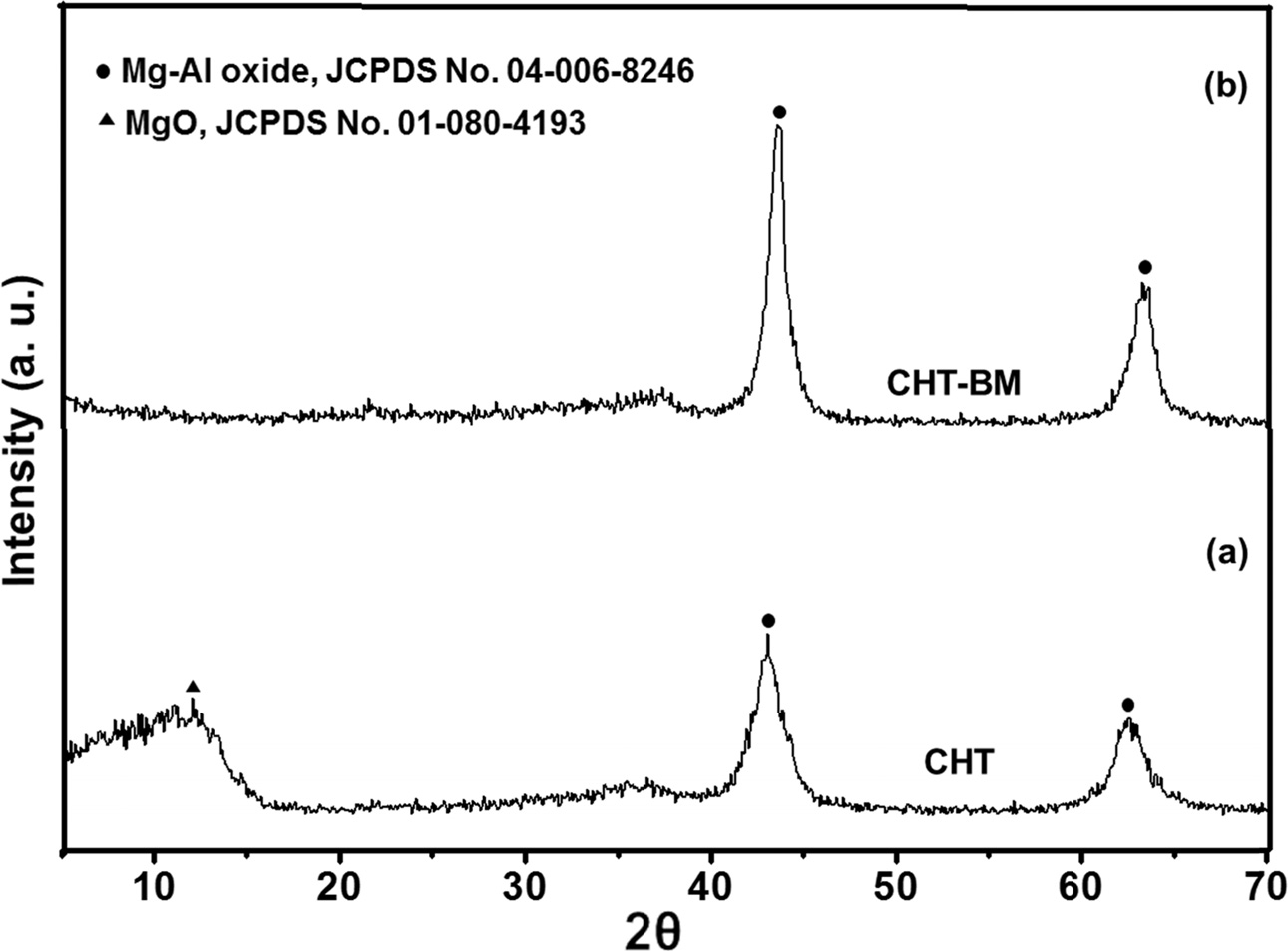
Fig. 1 shows the XRD patterns of the Mg-Al hydrotalcite powders prepared by the co-precipitation method
after calcination at 500 oC for 5 h (CHT), and the calcined
Mg-Al hydrotalcite powders after the ball-milling treatment for 2 hours
(CHT-BM). Granados and Serrano [35] reported that the hydrotalcite prepared by
co-precipitation before calcination showed a typical hydrotalcite pattern of
sharp and intense reflections at 11.7o, 23.2o, 60.6o
and 61.8o, at low values of 2θ angle, and less intense, asymmetric
reflections at higher angular values,
characteristic of a well-crystallized Mg-Al hydrotalcite compound, in agreement with the JCPDS card No. 41-1428,
of a double-layered material composed of a positively-charged brucite-like
layer and a negatively-charged interlayer, with the interlayer space typically occupied by water molecules and
anions for charge compensation. The
X-ray pattern of the Mg-Al
hydrotalcite calcined at 500 oC for 5 h (CHT) showed that its characteristic lamellar structure
disappeared, and that at this collapsed layer structure, only a well-dispersed
mixture of Mg and Al oxides was obtained with a high BET-specific surface area,
large total pore volume and a mesoporous diameter, as was revealed by N2
physisorption measurements (Table 1). It is well-know that, under calcination
at 400-500 oC, the Mg-Al hydrotalcite gradually loses interlaminar
water up to at approximately 200 oC; in the range of
200-500 oC, it is dehydroxylated
and decarbonated, allowing the formation of a well-dispersed mixture
of aluminum and magnesium oxides,
with a typical mixed oxide XRD trace obtained, where there are no peaks present
related to aluminium oxide, because aluminum oxide does not crystallize at the
relatively low temperature at which calcination is carried out.
Therefore, the X-ray diffraction pattern (Fig. 1) confirms
the formation of mixed oxides through calcination. When the calcined Mg-Al
hydrotalcite was ball-milled for 2 h, (labeled as the CHT-BM sample), the
presence of only a mixture of Mg and Al oxides was observed in this X-ray
pattern (Fig. 1), but with an increased cristallinity compared to
calcined hydrotalcite. The increased crystalline structure
can be attributed to the effect exerted on the adsorbent powders by the
ball-milling medium (stainless-steel balls), generating a nanostructured
material with a reduced particle size, which could improve the CO2 capture
[36]. It has been previously reported that smaller metal oxide particle sizes
provide a larger BET-specific surface area for exposure and, as a result, these
materials have a higher CO2 adsorption capacity.
Specifically, results have shown that both MgO and CaO-based
adsorbents prepared through solution-combustion synthesis and treated by
ball-milling, produced metal oxide particles with better textural and
structural properties for enhancing CO2 adsorption
capacity, proving that the mechanical process plays a
crucial role in CO2 adsorption behavior [20]. Thus, from this
stand-point, the nanostructured nature of the CHT-BM
sample should lead to greater reactivity and
increased adsorption effects, involving a large number of active
surface sites available for CO2 molecules.
Morphology
and EDS analysis
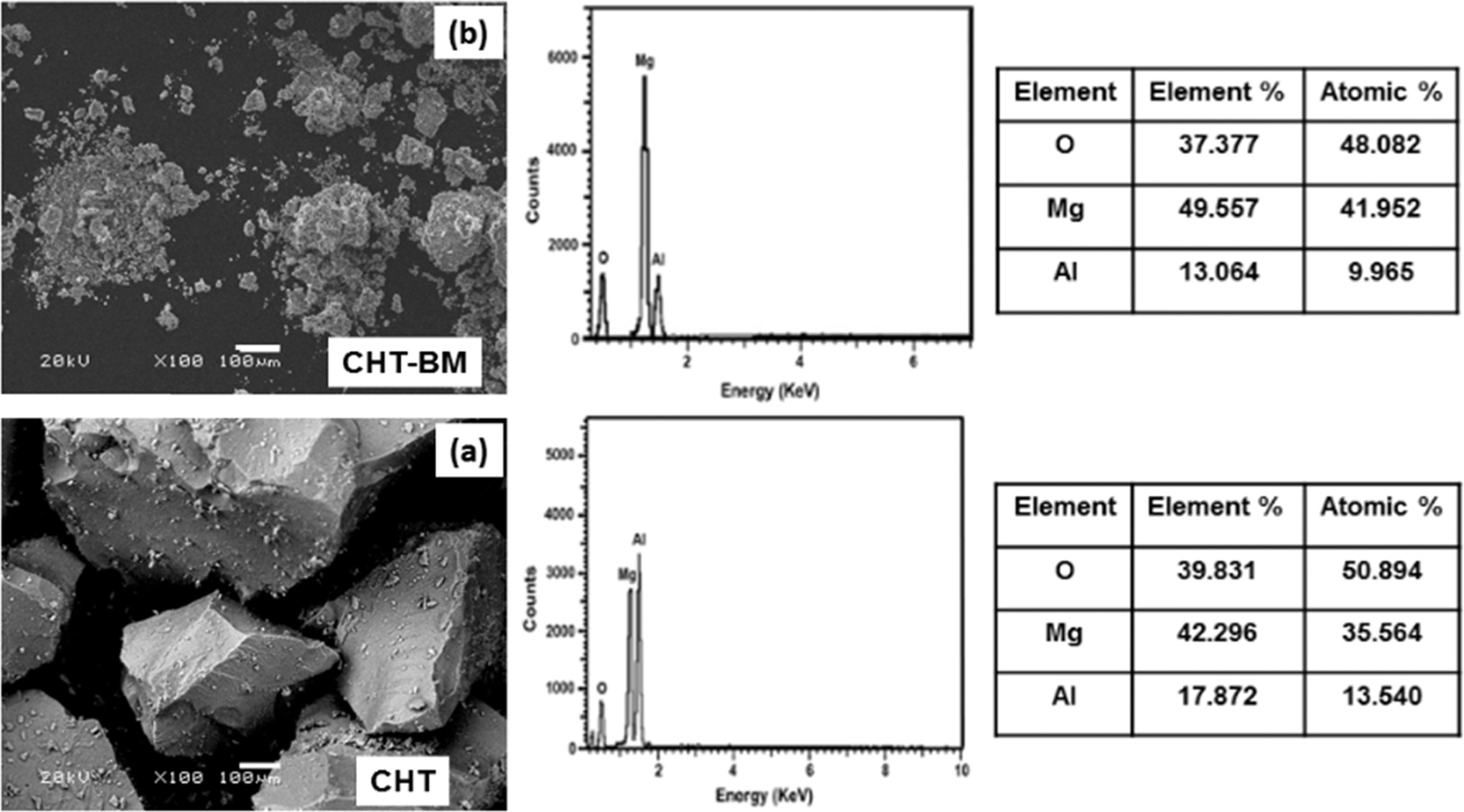
According to Rossi et al. [15], the formed mixture
of alkali Mg and Al oxides after hydrotalcite calcination, are strongly related
to the basic sites for the adsorption of acidified CO2
molecules. Yang and Kim [18] reported that the
basicity of hydrotalcite also depends on chemical composition (cation
type, M2+/M3+ ratio, anion type existing in the
interlayer) and activation conditions such as the degree of impregnation with
alkali metal carbonates. In consequence, the aluminum content directly affected the hydrotalcite´s CO2 adsorption
capacity, because the increase in
adsorbent Al content decreased the number of basic sites [15]. Therefore, based
on the obtained M2+/M3+
ratio, determined by energy-dispersive
X-ray spectroscopy (EDS) analysis (Fig. 2), it is clear that the calcined Mg-Al
hydrotalcite, followed by ball-milling
for 2 hours, exhibited the best features, and it was to be expected that this
material present the maximum CO2
adsorption capacity. Furthermore, in this study, it was also observed (by
SEM images) that the CHT-BM sample (Fig. 2) resulted in particle agglomeration
after mechanical ball-milling, and that important alterations of the original morphology and textural properties,
along with a predominant formation of particle agglomerates after using the ball-milling treatment, had an inverse
effect on CO2 adsorption capacity.
N2
adsorption-desorption isotherm analysis
On
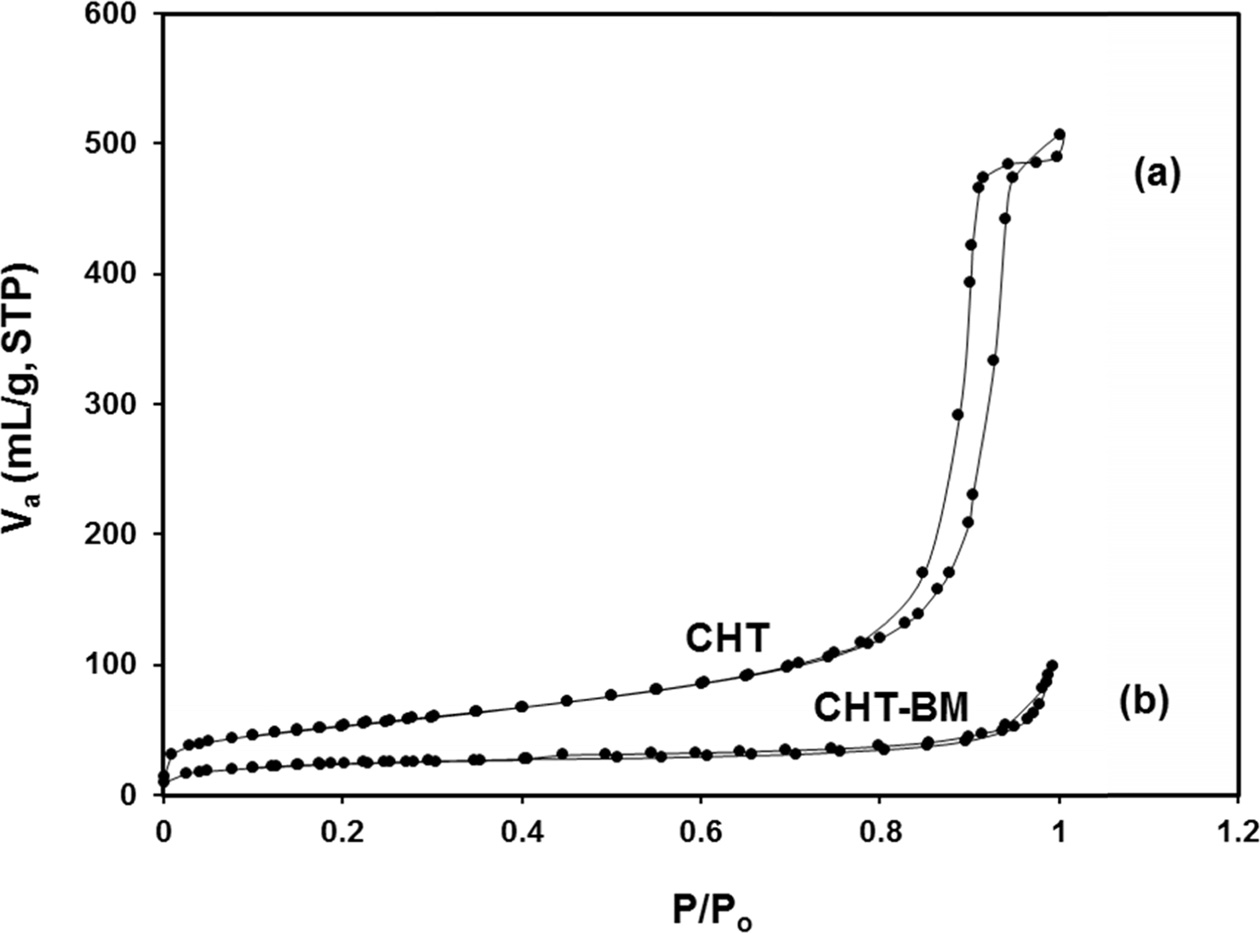
the other hand, the N2 adsorption-desorption isotherm results (Fig. 3), presented type IV isotherms of the studied samples, specifically for the CHT
sample, exhibited a marked low- adsorption hysteresis between the adsorption
and desorption curve, which, according to
the International Union of Pure and Applied Chemistry (IUPAC), is characteristic of mesoporous
materials. It is well-known that different adsorbent physicochemical characteristics such as BET-specific surface area, particle size, pore size, and pore volume, are
essential characteristics that play an
important role in CO2 adsorption
behavior, influencing the accessibility of CO2 in the pore structure
or adsorbent surface [36]; for example, adsorbents with a higher porosity have
a greater amount of pores, which determine a significant portion of the CO2
adsorption process, as well as physiadsorbents with small pores, which have
shown great potential as CO2
adsorbents. Indeed, CO2 adsorption increases as total pore volume increases, because a large pore volume
has more active sites for CO2, and experiences less diffusion resistance. According to
the main textural results (Table 1),
it can be seen that the Mg-Al
hydrotalcite, followed by ball-milling for 2 h (CHT-BM) does not have the most
significant physicochemical
characteristics, when compared to the calcined Mg-Al hydrotalcite sample (CHT)
without mechanical ball-milling treatment.
Pore
size distribution analysis
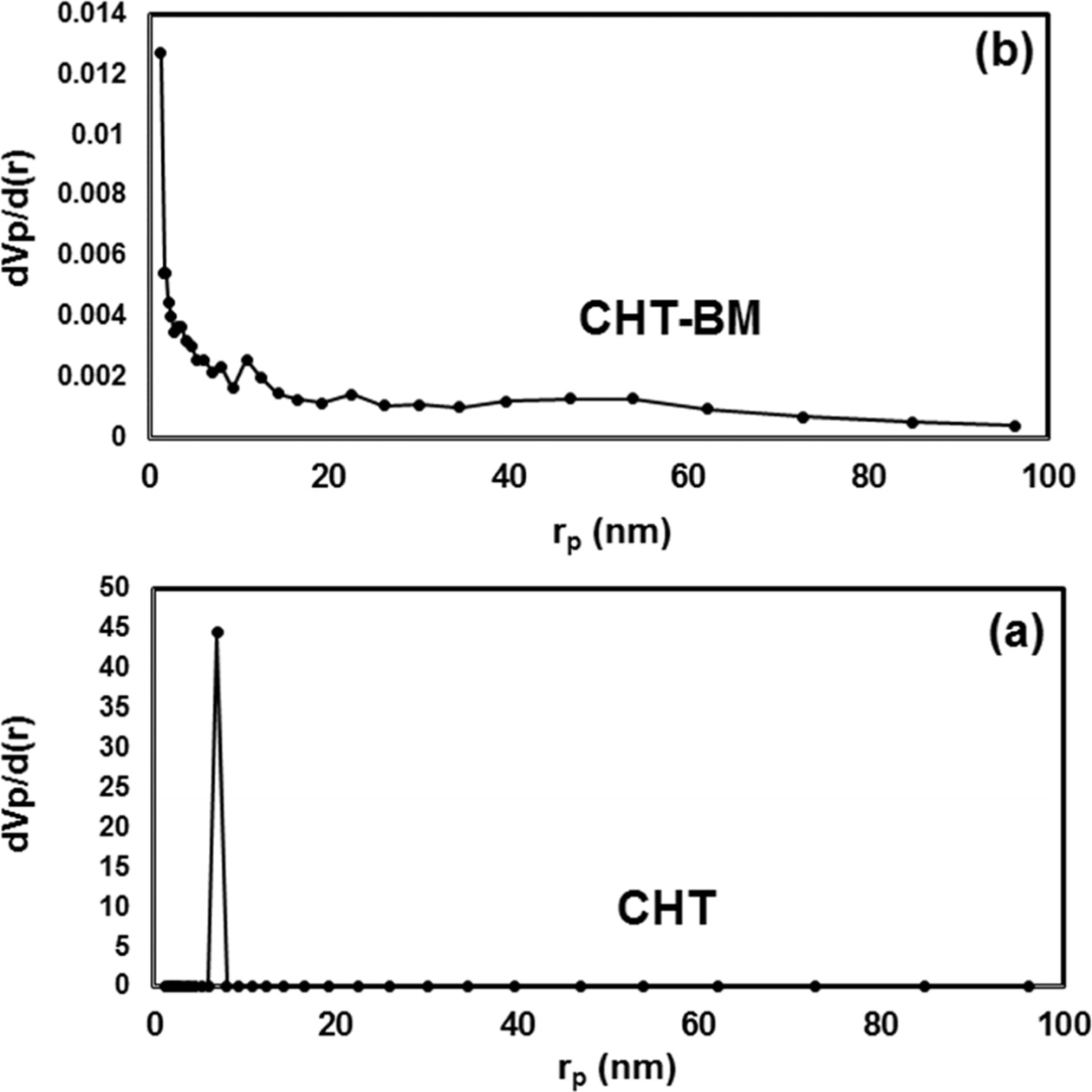
In addition, a significant reduction in pore size, from
10.76 nm to 1.21 nm, obtained by the Barrett-Joiner-Halenda (BJH) pore size distribution
method was also observed when the Mg-Al hydrotalcite was ball-milled (Fig. 4);
this sample showed a marked decrease in pore area (Ap) and total pore
volume (Vp) values (Table 1). It is known that the kinetic CO2
molecule diameter is 0.33 nm [37] and that the Mg-Al calcined hydrotalcite,
after ball-milling treatment, was drastically reduced in its pore diameter,
hence, the available pore space for CO2 did not lead to a better
diffusion of CO2 in the pore channels and decreased CO2
adsorption.
CO2
adsorption behavior
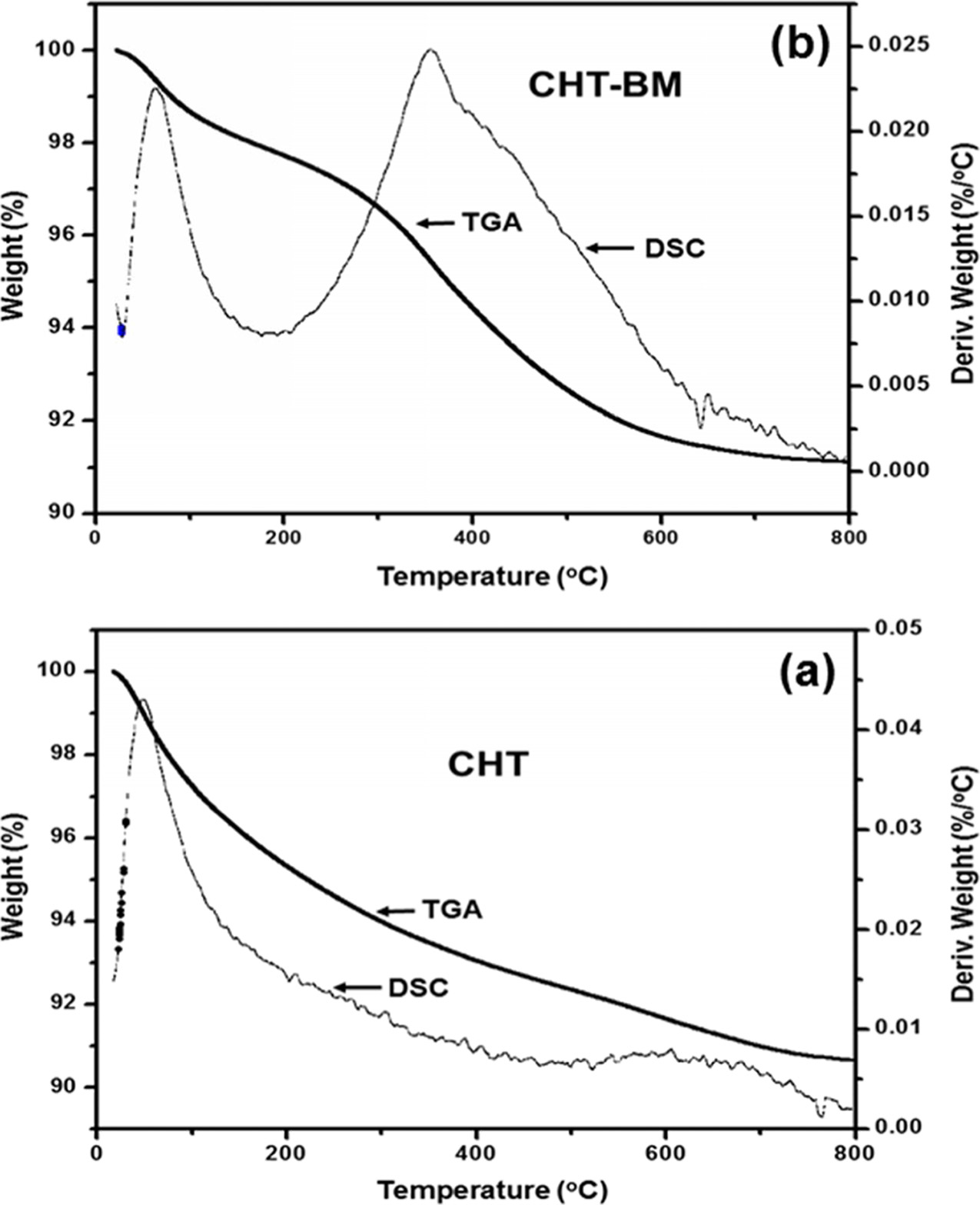
The CO2 adsorption behavior of CHT and CHT-BM samples
was examined. The obtained adsorption capacity values,
determined by thermogravimetric analysis and differential scanning calorimetry,
were different in both studied Mg-Al hydrotalcites. The TGA/DSC curves for CO2
desorbed from the studied hydrotalcite samples are shown in Fig. 5. It can be
observed that the CO2 adsorption capacity for CHT-BM was 2.018
mmol/g, which was 5.08% less than that observed value for CHT (2.126 mmol/g).
These obtained results are not consistent with those expected. The results
indicate that the ball-milling treatment negatively affects CO2
adsorption ability in the CHT-BM sample. It was expected that the
surface area would increase with mechanical ball-milling; however, it was
revealed that an important change occurred on the outer surface or inside the
CHT-BM sample. It is reasonable to expect that a mixed oxide derived from
calcined hydrotalcite, with considerable BET-surface area and high basicity,
would display an improved CO2 adsorption, and even more reasonable
to expect that the calcined Mg-Al hydrotalcite, treated by
ball milling (CHT-BM), would increase in its CO2 adsorption capacity
due to its nanometric scale and nature. Based on the above, the prepared CHT-BM
sample showed a decrease in its BET-surface area of 87.25 m2/g, in
comparison to the calcined Mg-Al hydrotalcite (191.97 m2/g), as well
as a decrease in its total pore volume, pore radius, as
can be seen in Table 1.
It can be clearly observed that the original small holes
and channels were blocked in the ball-milled calcined Mg-Al hydrotalcite
structure (CHT-BM). This alteration lead to a decrease in BET-surface area and
total pore volume, decreasing the effective reactive sites and reducing the
ability of the CHT-BM sample for CO2 capture. Also, it has been
widely reported that an appropriate pore size allows the diffusion of CO2
molecules inside of the adsorbent material; therefore, pore size can
inhibit or allow diffusion of CO2 molecules through the
hydrotalcite core, and certain pore sizes are ideal for CO2
adsorption. Consequently, an extended reduction in pore size can make the solid
adsorbent unsuitable for CO2 capture. In this regards, an average
particle-size reduction, resulting in the creation of fractures after
ball-milling, reduced the diffusion of CO2 molecules into the inner
framework of the CHT-BM and, as a consequence, the CO2
capacity of CHT-BM was decreased. In general, our results clearly
suggest that the agglomerate particle formation, a pore-size reduction,
decreased textural properties as a consequence of the
ball-milling treatment, which means that the treatment has negative effect over
the CO2 adsorption of calcined Mg-Al hydrotalcite. In that regard,
these significant results, demonstrate that CHT has the potential for CO2
capture, but that its efficiency is slightly decreased when this material is
ball-milled.
|
Fig. 1 XRD diffraction patterns of Mg-Al hydrotalcite samples prepared by the co-precipitation method; (a) no treatment (CHT) and (b) treatment with ball-milling for 2 h (CHT-BM). |
|
Fig. 2 SEM micrographs at 100X and EDS analysis of Mg-Al hydrotalcite samples prepared by the co-precipitation method; (a) no treatment (CHT) and (b) treated with ball-milling for 2 h (CHT-BM). |
|
Fig. 3 N2 adsorption-desorption isotherms of Mg-Al hydrotalcite samples prepared by the co-precipitation method; (a) no treatment (CHT), and (b) treated with ball milling for 2 h (CHT-BM). |
|
Fig. 4 Pore-size distributions by BJH method of Mg-Al hydrotalcite samples prepared by the co-precipitation method, with (a) no treatment (CHT) and (b) after treatment with ballmilling for 2 h (CHT-BM). |
|
Fig. 5 TGA-DSC curves of CO2 adsorption of Mg-Al hydrotalcite samples at 200 oC and 1 atmosphere: (a) calcined Mg-Al hydrotalcite (CHT) and (b) calcined Mg-Al hydrotalcite after ball-milling treatment for 2 h (CHT-BM). |
|
Table 1 Main textural properties of calcined Mg-Al hydrotalcite (CHT), and calcined Mg-Al hydrotalcite after ball-milling treatment for 2 h (CHT-BM). The samples were previously degassed with N2 at 300 oC for 2 h. |
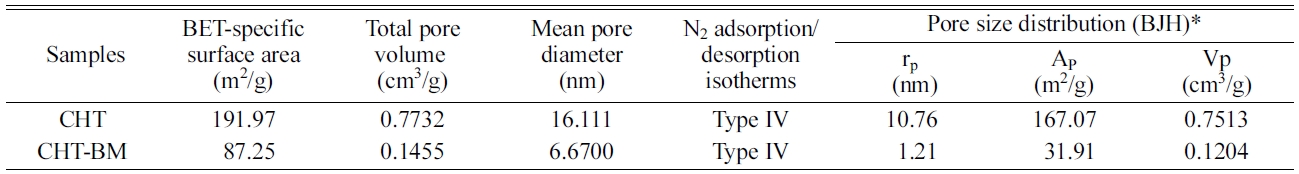
*Barrette-Joyner-Halenda (BJH) method |
In this study, we investigated the effect of ball-milling
over the CO2 adsorption capacity of calcined Mg-Al hydrotalcite,
recognizing that this treatment is well-studied for the preparation of
nanostructured materials that should improve CO2
adsorption. However, in this work, it was demonstrated
that the ball-milling process is not considered a viable alternative for the
improvement of the CO2 absorption of calcined Mg-Al hydrotalcite,
due to its high particle aggregate formation, decreased
BET-surface area and pore size. Thus, based on all the aforementioned results,
it was concluded that the ball-milling process does not play a crucial role in
the obtainment of hydrotalcite compounds with better textural and structural
properties for enhancement of CO2 adsorption, and that
the calcined Mg-Al hydrotalcite treated by ball milling for 2 h does
not appear to be useful for large-scale CO2 capture technologies,
but certainly efficient when not treated by ball-milling.
This work was supported by the National Institute of
Nuclear Research (ININ), México, through research project CB-706, stages I to
III.
- 1. A. Yamasaki, J. Chem. Eng. Jpn. 36[4] (2003) 361-375.
-

- 2. D. Aaron and C. Tsouris, Sep. Sci. Technol. 40[1-3] (2005) 321-348.
-

- 3. S.Choi, J.H. Drese, and C.W. Jones, ChemSusChem 2[9] (2009) 796-854.
-

- 4. M.S. Shafeeyan, W.M.A.W. Daud, A. Houshmand, and A. Shamiri, J. Anal. Appl. Pyrol. 89[2] (2010) 143-151.
-

- 5. N. Chalal, H. Bouhali, H. Hamaizi, B. Lebeau, and A. Bengueddach, Micropor. Mesopor. Mat. 210 (2015) 32-38.
-

- 6. C. Chen, D. Park, and W. Ahn, Appl. Surf. Sci. 292 (2014) 63-67.
-

- 7. R. Dawson, A.I. Cooper, and D.I. Adams, Polym. Int. 62[3] (2013) 345-352.
-

- 8. H. Hedin, L. Andersson, L. Bergström, and J. Yan, Appl. Energy 104 (2013) 418-433.
-

- 9. N.A. Rashidi, S. Yusup, and A. Borhan, Procedia Eng. 148 (2016) 630-637.
-

- 10. K. Wang, X. Gou, P. Zhao, L. Zhang, and C. Zheng, Chem. Eng. J. 173 (2011) 158-163.
-

- 11. F. Granados-Correa, J. Bonifacio-Martínez, H. Hernández-Mendoza, and S. Bulbulian, J. Air Waste Manage. Assoc. 66[7] (2016) 643-654.
-

- 12. A. Miyata, Clays Clay Miner. 31[4] (1983) 305-311.
-

- 13. G. Mao, M. Tsuji, and Y. Tamaura, Clays Clay Miner. 41[6] (1993) 731-737.
-

- 14. F. Granados-Correa, J. Vilchis-Granados, M. Jiménez-Reyes, and L.A. Quiroz-Granados, J. Chem., 2013 Art. ID 751696, 1-9 pages.
-

- 15. T.M. Rossi, J.C. Campos, and M.M.V.M. Souza, Adsorption 22[2] (2016) 151-158.
-

- 16. F. Cavani, F. Trifiro, and A. Vaccari, Catal. Today 11[2] (1991) 173-301.
-

- 17. E.L.G. Oliveira, C.A. Grande, and A.E. Rodrigues, Sep. Purif. Technol. 62[1] (2008) 137-147.
-

- 18. J. Yang and N. Kim, Korean J. Chem. Eng. 23[1] (2006) 77-80.
-

- 19. M.H. Halabi, M.H.J.M. de Croon, V.D. Schaaf, P.D. Cobden, and J.C. Schouten, Int. J. Hydrogen Energy 37[5] (2012) 4516-4525.
-

- 20. E. Gutiérrez-Bonilla, F. Granados-Correa, V. Sánchez-Mendieta, and R.A. Morales-Luckie, J. Environ. Sci. 57 (2017) 418-428.
-

- 21. V. Manovic and E.J. Anthony, Int. J. Environ. Res. Public Health 7[8] (2010) 3129-3140.
-

- 22. F. Raganati, P. Ammendola, and R. Chirone, Powder Technol. 268 (2014) 347-356.
-

- 23. F.N. Ridha, V. Manovic, A. Macchi, and E.J. Anthony, Appl. Energy 140 (2015) 297-303.
-

- 24. E. Ordoñez-Regil, F. Granados-Correa, En. Ordoñez-Regil, and M.G. Almazán-Torrez, Environ. Technol. 36[2] (2015) 188-197.
-

- 25. J.M. Valverde, P.E. Sanchez-Jimenez, and L.A. Perez-Maqueda, Environ. Sci. Technol. 48[16] (2014) 9882-9889.
-

- 26. I.J. Son, K.I. Na, W. Kim, J.W. Lim, J.M. Doh, and J. Yoon, J. Ceram. Process. Res. 13 [16] (2012) 272-277.
- 27. L. Nikzerd, T. Ebadzadeh, M.R. Vaezi, and A. Tayebifard, J. Ceram. Process. Res. 13[5] (2012) 590-594.
- 28. W. Janusz, S. Khalameida, V. Sydorchuk, E. Skwarek, V. Zazhigalov, and Z.J. Skubiszewska, Adsorption 16[4-5] (2010) 333-341.
-

- 29. J. Qu, L. Sha, C. Wu, and Q. Zhang, Nanomaterials 9[1] (2019) 80.
-

- 30. L.N. Stepanova, O.B. Belskaya, O.N. Baklanova, A.V. Vasilevich, and V.A. Likholobov, Procedia Eng. 152 (2016) 672-680.
-

- 31. J. Qu, Q. Zhang, X. Li, X. He, and S. Song, Appl. Clay Sci. 119 (2016) 185-192.
-

- 32. J. Mi, X. Chen, Q. Zhang, Y. Zheng, Y. Xiao, F. Liu, C. Au, and L. Jiang, Chem. Commun. 55[63] (2019) 9375-9378.
-

- 33. M.R. Othman, Z.H. Martunus, and W.J.N. Fernando, Appl. Organomet. Chem. 23[9] (2009) 335-346.
-

- 34. T. Sato, H. Fujita, T. Endo, and M. Shimada, J. React. Solids 5[2-3] (1998) 218-219.
-

- 35. F. Granados Correa and J. Serrano Gómez, J. Radianal. Nucl. Chem. 268[1] (2006) 95-101.
-

- 36. F. Granados-Correa, J. Bonifacio-Martínez, and H. Hernández-Mendoza, Water Air Soil Pollut. 226[9] (2015) 1-10.
-

- 37. X. Xu, X. Zhao, L. Sun and X. Liu, J. Nat. Gas Chem. 18[2] (2009) 167-172.
-

 This Article
This Article
-
2019; 20(6): 597-602
Published on Dec 31, 2019
- 10.36410/jcpr.2019.20.6.597
- Received on Jul 19, 2019
- Revised on Oct 4, 2019
- Accepted on Oct 7, 2019
 Services
Services
- Abstract
experimental procedure
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- F. Granados-Correa
-
Instituto Nacional de Investigaciones Nucleares, Departamento de Química, A.P. 18-1027, Col. Escandón, Delegación Miguel Hidalgo, C.P. 11801, Ciudad de México, México
Tel : + 55-53297200 Fax: + 55-53297301 - E-mail: francisco.granados@inin.gob.mx






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.