- Effect of pyrolysis temperature on heat-generating behavior and morphology of SiC fiber mats
Young Jun Jooa,b, Kwang Youn Chob,* and Cheol Jin Kima,*
aResearch Institute of Green Energy Convergence Technology, Gyeongsang National Univ., Jinju 52828, Korea
bCeramic Fiber & Composites Center, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Korea
Silicon carbide (SiC) fibers fabricated by melt-spinning, curing, and pyrolysis of polycarbosilane (PCS) as a ceramic precursor were used to fabricate the heat-generating mats by interaction with microwave. In this study, the effect of pyrolysis temperature on heat-generating temperature of SiC fiber mats was investigated to optimize the performance of mats via microstructure control in the fiber. SiC fibers synthesized at high pyrolysis temperature contained a relatively low oxygen content due to the residual oxygen release in the form of SiO and CO gases. The optimization of the elemental composition in SiC fiber by minimizing oxygen level enable the maximum heat-generating temperature and life-time of the SiC fiber mats under microwave. Also, the polymer-derived SiC fiber mats exhibited both oxidative- or thermal-degradation due to the non-uniform heat-generating by the edge effect under microwave.
Keywords: SiC fiber, Microwave, Heat-generating, Morphology, Degradation
Inorganic fibers, which are largely classified into oxide fibers and non-oxide fibers, have been used mainly in extreme environments due to its high heat resistance and specific strength [1, 2]. In particular, silicon carbide (SiC) fibers having the highest oxidation resistance (> 1,300 ℃) among inorganic fibers are manufactured as SiC/SiCf composites [3, 4], and are being applied to the various extreme applications including rocket nozzles and engines in the aerospace industries and disc brake in the sports industries [5-7].
Since the SiC fibers are generally used as a reinforcing material of the composite materials, the development of polymer-derived SiC fibers have been focused on improving the heat-resistant characteristics. Three generations of SiC fibers were developed from Ube Industries Ltd. (Tyranno Lox M, Tyranno ZMI, and Tyranno SA) [8-10] and Nippon Carbon Co. Ltd. (Nicalon, Hi-Nicalon, and Hi-Nicalon Type S) [11-13] in japan, and theses fibers had strength retention temperature of 1,100 ℃, 1,500 ℃, and 1,700 ℃, respectively. As a result, the industrial application field of SiC fiber still has a limit to be used as a structural material.
Polymer precursors are converted from polymers to ceramics through heat treatment in an inert atmosphere. However, the ceramic conversion without the curing process has a low ceramic yield of about 50 ~ 60 and fiber shape is broken by decomposition into gas form. In a previous study [14, 15] by Korea Institute of Ceramic Engineering and Technology (KICET), the chemical vapor curing (CVC) method using iodine was developed as the curing process for converting polymer to ceramic. Iodine curing has the advantage of rapidly inducing cross-linking of PCS having a low melting point. Also, SiC fibers converted by iodine curing method were fabricated in the form of multi-layered mats, which showed heat-generating behavior up to 1,100 ℃ under microwave [16, 17]. This fiber mats also reached a maximum temperature in about 30 sec. Therefore, a heat treatment system with SiC fiber mats under microwave can significantly reduce the time and cost of the thermal production process.
The study on heat-generating mechanism of SiC fiber under microwave has not been fully understood. In general, the interaction between materials and microwave in non-magnetic materials is largely divided into dipole loss and conduction loss, and the dielectric properties is very important factor for microwave-absorption. H. Tian et al. reported that the electrical conductivity and dielectric properties of polymer-derived SiC-based ceramics depend on the content and degree of pyrolytic carbon [18]. Also, B. Wang et al. reported that dielectric loss of Si-C-Ti-B fibers increases with the annealing temperatures [19]. However, previous papers did not mention the heat-generating behavior of SiC fibers via microwave-absorbing.
In this study, the polymer-derived SiC fibers were prepared with the different pyrolysis temperatures and the heat-generating behavior under microwave irradiation was investigated. The interaction of SiC fibers and microwave induces heat generation by itself at a temperature up to 1,100 ℃ or higher, thereby causing oxidation and degradation of the fiber. Therefore, the microstructure of SiC fibers before and after the microwave irradiation was also analyzed to understand the thermal behavior under microwave irradiation.
Preparation of polymer fibers
Polycarbosilane (PCS) as a ceramic precursor was purchased from TBMTech. Co. Ltd. (Korea). PCS, having a weight average molecular weight (Mw) of 4,000 and polydispersity (Dp) of 2.5 was melt-spun using single-hole spinning machine at 243 ℃ into the fiber. PCS green fibers have a 20-25 μm diameter.
Conversion polymer fibers into ceramic fibers
The chemical vapor curing (CVC) method with iodine metal [14, 15] was adopted to fabricate the infusible PCS green fibers due to the low molecular weight and melting point of a ceramic precursor. The PCS green fibers and iodine metal of 1:1 ratio were put into graphite crucible, and then heat-treated at 180 ℃ for 2 h under the low pressure. As a result of chemical vapor curing (CVC), the PCS green fibers of white color changed to pale-yellow. Lastly, the cured PCS fibers were converted to SiC fibers through pyrolysis in an argon atmosphere. The mechanical and electrical properties of SiC fibers depend on the pyrolysis temperature, which affects the microstructure and composition of the fiber. In this experiments, the pyrolysis temperature was controlled to 1,300 ℃, 1,350 ℃, 1,400 °C, and 1,450 ℃ for 2 h, respectively.
Measurements
The morphological and elemental analysis of SiC fibers were analyzed using field-emission electron microscopy (FE-SEM, JSM-7610F, JEOL, Japan) and energy dispersive spectroscopy (EDS, INCA x-sight, Oxford Instruments, UK). The platinum (Pt) was coated on the fiber for 120 s before the observation. The mechanical strength of SiC fiber was measured via tensile strength measurement system (load cell: UMG-G200, DACELL, Korea). The ends of the single-filament with a length of 40 were fixed using instantaneous adhesives on the paper, and then the prepared samples were loaded on the measuring instrument. The paper used to fix the fiber was removed before the measurement. The microwave generating system with 2.45 GHz frequency and controllable current intensity (DAEHO I&T Co. Ltd., Korea) was used, which is a stirrer type for uniform microwave irradiation. Infrared camera (HotFind-LR, SATIR, Europe) was used to investigate the maximum heat-generating temperature and heating rate of SiC fiber mats under the microwave. The process temperature control rings (PTCR, PTCR-HTH, Ferro Co., France) was used to investigate heat-generating performance as heating element.
The characteristics of fabricated SiC fibers
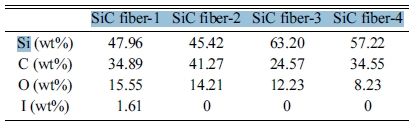
Although the SiC fibers derived from the polymer mainly are composed of silicon and carbon, the fibers usually contain a small amount of impurities depending on the type of precursor and the curing method. In this study, the polymer-derived SiC fibers were prepared using the iodine curing method, which allow PCS to cross-link at low temperatures. Therefore, the SiC fibers fabricated by this method generally contain impurities such as oxygen and iodine because the role of iodine assists the cross-linking of oxygen and PCS at low temperatures [14]. The iodine-cured PCS fibers were pyrolized at 1,300 ℃, 1,350 ℃, 1,400 ℃, and 1,450 °C, respectively, and were labeled SiC fiber-1, SiC fiber-2, SiC fiber-3, SiC fiber-4. Before heat-generating test under microwave, these fibers were analyzed by FE-SEM, XRD, and tensile strength measurement.
Fig. 1 shows the surface and cross-section of SiC fibers fabricated at each temperature. The surface of the fibers was smooth without pores or crack. However, the component content of the fibers changed as the heat treatment temperature increased and was summarized in Table 1. The iodine still remained in the heat-treated SiC fibers at 1,300 ℃ for 2 h, but was completely removed in SiC fiber-2, SiC-3, and SiC-4. The oxygen also tended to decrease continuously as the pyrolysis temperature increased, and the average diameter of SiC fibers was shrunk from 17.28 μm to 14.63 μm due to the decomposition reaction.
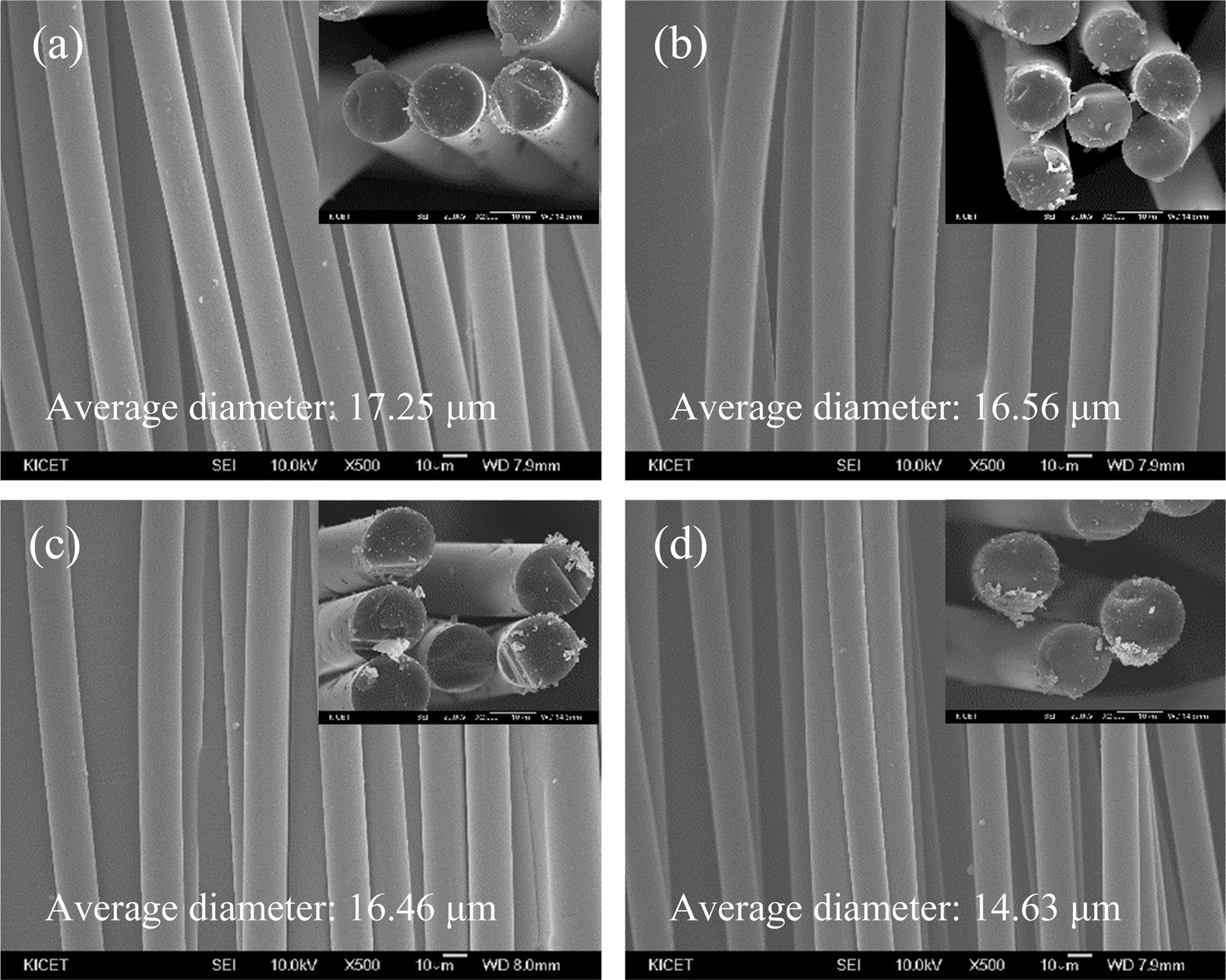
The cross-sectional SEM images at high magnification (Fig. 2) show the microstructure difference of SiC fiber-1 and SiC fiber-4 depending on the pyrolysis temperature. The SiC fibers pyrolized at 1,300 ℃ for 2 h showed relatively dense microstructure, whereas the SiC fibers pyrolized at 1,450 ℃ for 2 h showed grain growth and nano-size pores. It is generally known that the amorphous SiO2 phase formed by oxygen impurity begins to decompose at 1,522 ℃. At lower temperatures, however, SiC fibers undergo crystal growth of SiC phase because of the released CO gas by the main reaction of Si-O bond and excess carbon at the interface. Therefore, this reaction is consistent with the tendency of oxygen to decrease continuously in the EDS results and causes nano-pores as shown in Fig. 2(b).
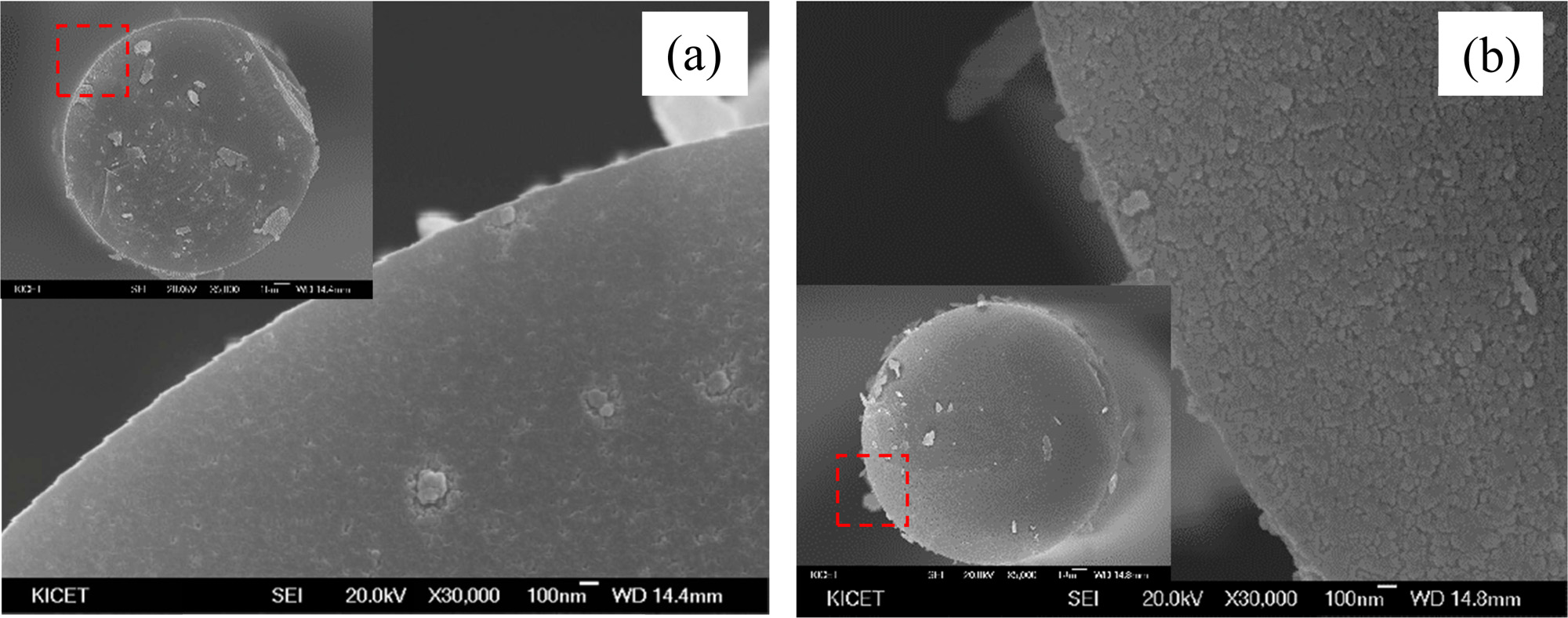
As described above, the fabrication temperature affects the strength of the fibers because of the grain growth of SiC and decomposition in the form of SiO and CO gases. Fig. 3 shows the mechanical strength of SiC fibers fabricated at different temperatures. The tensile strength of the fibers appears to decrease steadily with increasing pyrolysis temperature, but the difference in the average tensile strength of the samples fabricated at 1,300 ℃ and 1,450 ℃ was about 0.3 GPa. Therefore, the heat-generating characteristics and degradation behaviors of SiC fiber mats at different composition ratios were investigated under microwave.
Heat-generating and degradation behaviors of SiC fiber mats under microwave
In previous studies [16, 17], SiC fiber mats under microwave showed the different heat-generating characteristics depending on the thickness of fiber mats. Therefore, the same amount of cured PCS fibers was stacked in the graphite mold, and then, the cured PCS fiber mats consisting of five layers were heat-treated at 1,300 ℃, 1,350 ℃, 1,400 ℃, and 1,450 ℃ in argon atmosphere under the same load. The heat-generating characteristics of the samples prepared at each heat treatment temperature under microwave was measured using an infrared camera and process temperature control rings (PTCR). Since PTCR shrinked to a specific size due to total heat from convection, radiation, and conduction, the temperature inside the furnace could be measured by converting the amount of shrinkage into temperature.
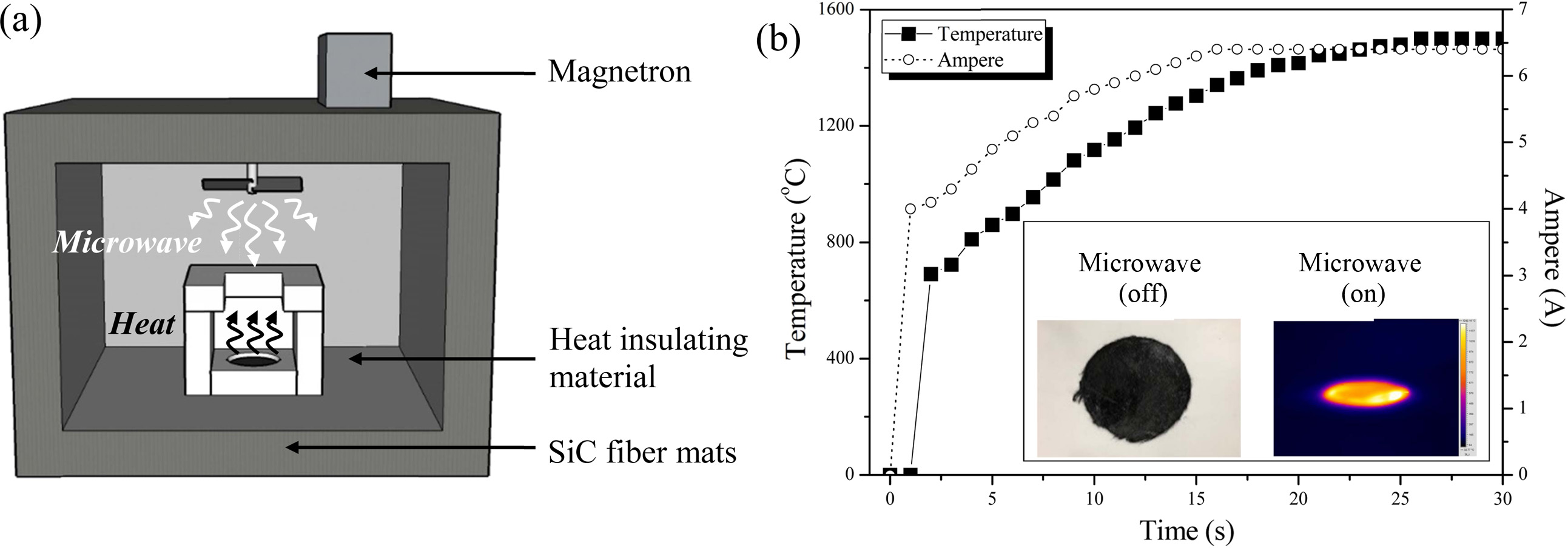
Fig. 4(a) is a schematic drawing of a microwave generation system (2.45 GHz) with the current in the range of 0 to 6.5 A. The system adopted stirrer type that can irradiate the material with uniform microwaves, thus allowing a relatively uniform and constant microwave absorption in all parts of the sample body. As shown in Fig. 4(b), the maximum temperature of SiC fiber mats reached only in 26 seconds depending on the applied current.
SiC fiber mats and PTCR were placed inside the insulating material, and the microwave was irradiated for 2 h to investigate the heat-generating performance and degradation behavior of the fiber mats for a long time. As shown in Table 2, the maximum heat-generating temperature of SiC fiber mats increased slightly with increasing pyrolysis temperature. Y. Sasaki et al. reported that clusters of excess carbon present in the polymer-derived SiC fibers tend to grow at heat treatment temperature of about 1,400 ℃ and diminished above 1,500 ℃ [20]. Since the heat treatment up to about 1,500 ℃ induces the growth of carbon cluster, the electrical conductivity and dielectric properties of SiC fibers changed and microwave-absorption increased.
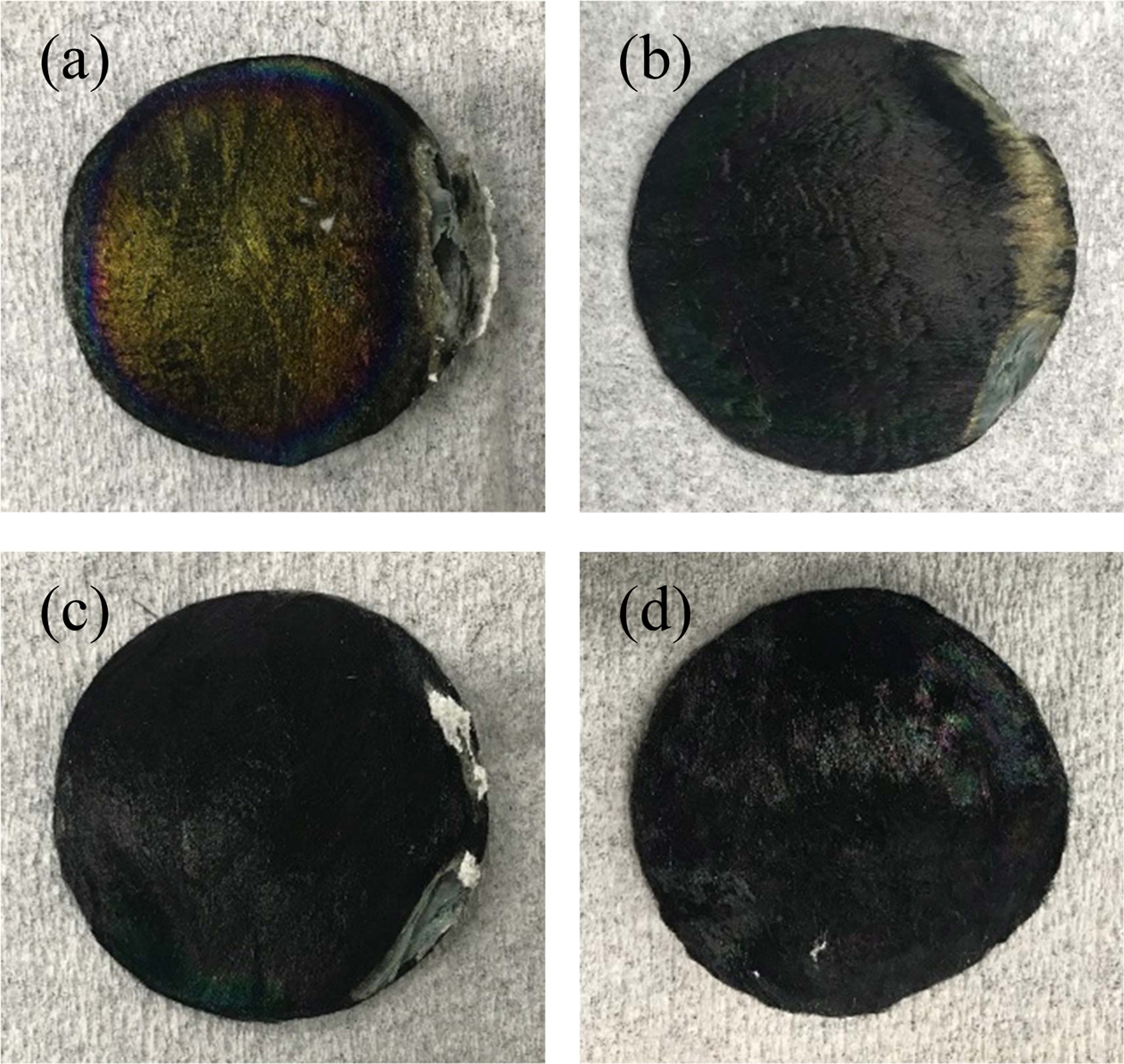
Fig. 5 shows the morphology of SiC fiber mats fabricated at 1,300 ℃, 1,350 ℃, 1,400 ℃, and 1,450 °C after heat-generating for 2 h under microwave. All the samples began to degraded in a specific direction due to the edge effect of the microwave concentrated at the end or corner of the material body. However, the SiC fiber mats-4, which had the highest heat-generating capability, showed no change in color and maintained a circular shape.
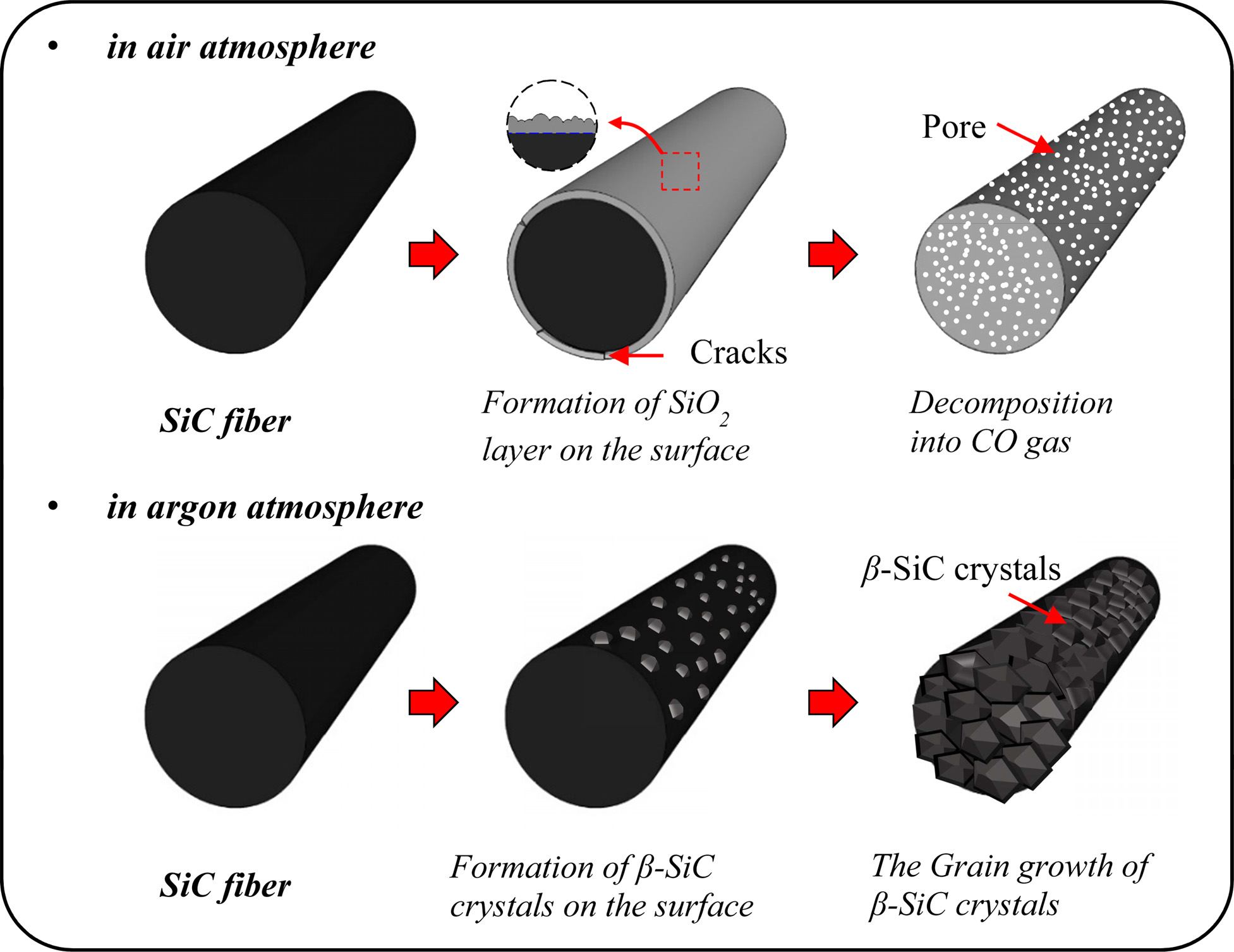
Amorphous SiC fibers containing oxygen as an impurity exhibited different decomposition behaviors depending on the atmosphere at high pyrolysis temperature as shown in Fig. 6. In the air atmosphere (1), SiC fibers underwent phase transition to SiO2 by reaction with oxygen and continuously released CO gas that forms the pores on the surface and inside the fibers [21, 22]. On the other hand, in the inert atmosphere (2-3), SiC fibers decomposed into SiC and free carbon, accompanied with the release of SiO and CO gases. Then, the reaction of SiO and CO gases formed SiC crystals on the fiber surface [23, 24]. The main reaction formula for decomposition behavior of SiC fibers is shown below.
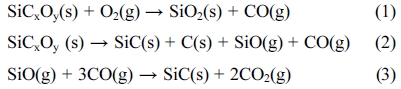
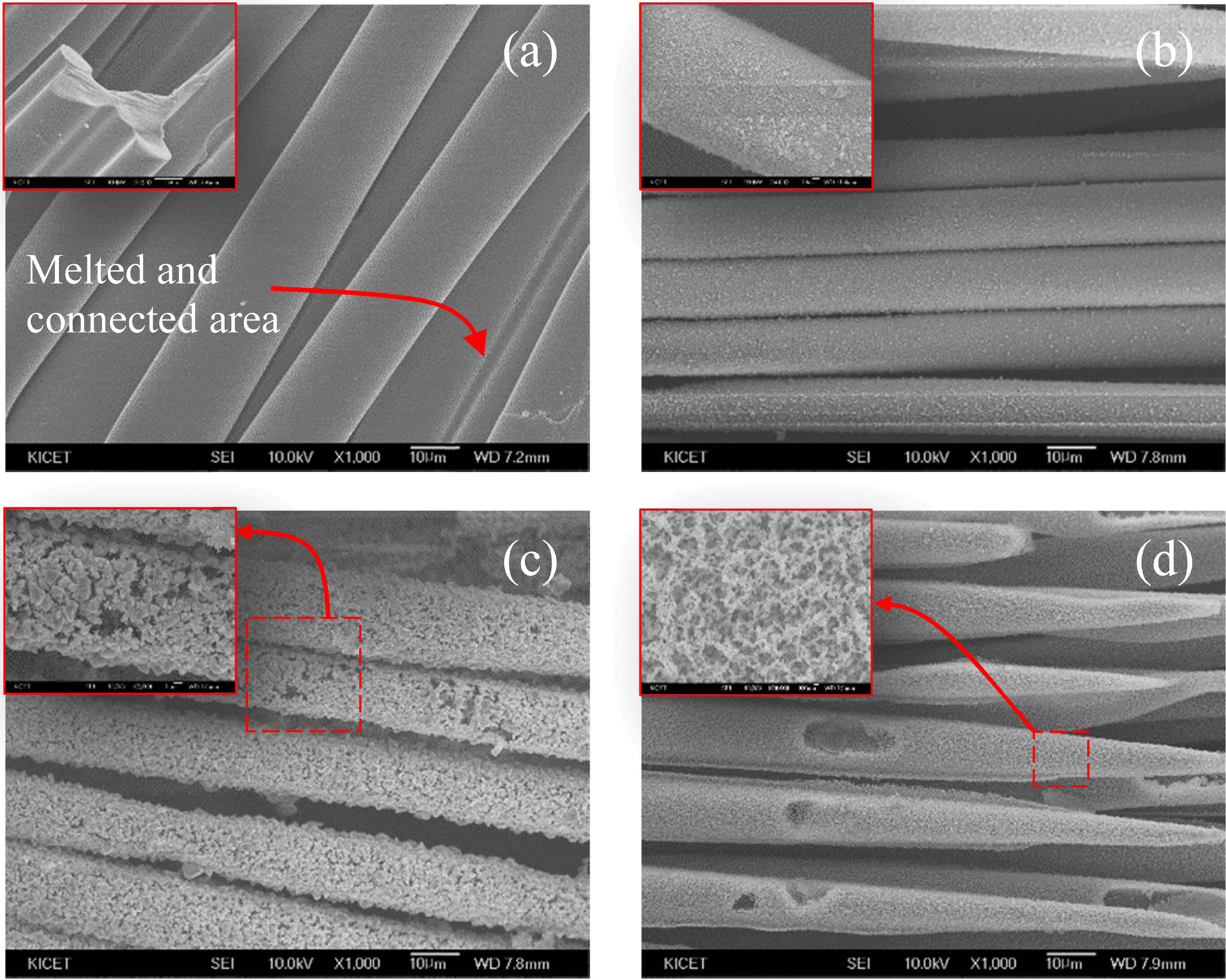
Fig. 7(a) shows the end area of SiC fiber mats-4, and Fig. 7(b-d) shows the three areas from the core to the end of SiC fiber mats-1. As mentioned in Fig. 6, since the decomposition reaction and the resultant morphology of SiC fibers changed with the pyrolysis atmosphere, the morphology of SiC fibers after microwave assisted heating for 2 h in Fig. 7 could be classified into four types including the oxidative degradation and thermal degradation. Fig. 7(a) and (d) are similar to the oxidative degradation behavior and Fig. 7(b) and (c) are similar to the thermal degradation behavior.
The regions with different morphologies in SiC fiber mats were investigated by EDS analysis to verify the oxidative or thermal degradation. Fig. 7(a), (b), (c), and (d) are labeled Region 1, Region 2, Region 3, and Region 4, respectively. In region 1 and 4, carbon was not detected or greatly decreased because carbon was decomposed into CO gas by oxidative degradation. On the other hand, region 2 and 3 exhibited the same morphology as thermal degradation behavior and consisted of silicon and carbon with little oxygen content. SEM and EDS results showed that different degradation could occur in the fiber mats due to the uneven heat-generating characteristics under even stirrer-type microwave heating system in this study.
|
Fig. 1 SEM images of SiC fiber fabricated at (a) 1,300 ℃, (b) 1,350 ℃, (c) 1,400 ℃, and (d) 1,450 ℃ for 2 h in an inert atmosphere. |
|
Fig. 2 The cross-sectional SEM images of (a) SiC fiber-1 and (b) SiC fiber-4. |
|
Fig. 3 Tensile strength of polymer-derived SiC fiber fabricated at different temperatures. |
|
Fig. 4 (a) A schematic drawing of a microwave generator with SiC fiber mats and (b) time-temperature graph of SiC fiber mats under microwave. |
|
Fig. 5 Photographs of SiC fiber mats after the microwave-assisted heating for 2 h: (a) SiC fiber mats-1, (b) SiC fiber mats-2, (c) SiC fiber mats-3, and (d) SiC fiber mats-4. |
|
Fig. 6 Degradation behavior of polymer-derived SiC fibers in the different pyrolysis atmospheres. |
|
Fig. 7 SEM images of (a) SiC fiber mats-4 and SiC fiber mats-1 degraded under microwave for 2 h. |
|
Table 2 Heat-generating temperature of SiC fiber mats calculated from shrinkage of PTCR. |

Silicon carbide fiber mats, which can generate heat under microwave, were fabricated at different pyrolysis temperatures. As the pyrolysis temperature increased, the strength of the fibers decreased relatively, and the maximum heat-generating temperature under microwave increased due to the growth of carbon cluster in the SiC fibers. Also, SiC fiber mats with low oxygen content maintained its color and shape despite heat-generating up to 1,560 ℃ under microwave for 2 h. In other words, the heat-generating temperature and life-time of SiC fiber mats were increased by controlling the pyrolysis temperature.
This work was supported by the technology development program (S2646118) and funded by ministry of SMEs and Startups (MSS, Korea).
- 1. C.A. Finch, in “Encyclopedia of Textiles, Fibers, and Nonwoven Fabrics” (Wiley-Interscience, 1984) p. 438-450.
-

- 2. T.F. Cooke, J. Am. Ceram. Soc. 74[12] (1991) 2959-2978.
-

- 3. J.Y. Park, S.M. Kang, and W.J. Kim, J. Ceram. Process. Res. 10[3] (2009) 364-366.
- 4. G.Y. Gil and D.H. Yoon, J. Ceram. Process. Res. 12[4] (2011) 371-375.
- 5. B. Budiansky, and J. W. Hutchinson, J. Mech, Phys. Solids. 34[2] (1986) 167-189.
-

- 6. R. Naslain, Compos. Sci. Technol. 64[2] (2004) 155-170.
-

- 7. S. Schmidt, S. Beyer, H. Knabe, H. Immich, R. Meistring, and A. Gessler, Acta. Astronautica 55[3-9] (2004) 409-420.
-

- 8. T. Yamamura, T. Ishikawa, M. Shibuya, T. Hisayuki, and K. Okamura, J. Mater. Sci. 23[7] (1988) 2589-2594.
-

- 9. H. Yamaoka, T. Ishikawa, and K. Kumagawa, J. Mater. Sci. 34[6] (1999) 1333-1339.
-

- 10. T. Ishikawa, Y. Kohtoku, K. Kumagawa, T. Yamamura, and T. Nagasawa, Nature 391[19] (1998) 773-755.
-

- 11. H. Ichikawa, H. Teranishi, and T. Ishikawa, J. Mater. Sci. 6[4] (1987) 420-422.
-

- 12. M. Takeda, J.-I. Sakamoto, Y. Imai, and H. Ichikawa, Compos. Sci. Technol. 59[6] (1999) 813-819.
-

- 13. M. Takeda, A. Saeki, J.-I. Sakamoto, Y. Imai, and H. Ichikawa, J. Am. Ceram. Soc. 83[5] (2000) 1063-1069.
-

- 14. J.S. Hong, K.Y. Cho, D.G. Shin, J.I. Kim, S.T. Oh, and D.H. Riu, J. Mater. Chem. A 2[8] (2014) 2781-2793.
-

- 15. J.S. Hong, K.Y. Cho, D.G. Shin, J.I. Kim, and D.H. Riu, J. Appl. Polym. Sci. 132[47] (2015) 42687.
-

- 16. K.E. Khishingbayar, Y.J. Joo, and K.Y. Cho, J. Korean Ceram. Soc. 54[6] (2017) 499-505.
-

- 17. K.E. Khishingbayar, J.M. Seo, and K.Y. Cho, J. Korean Ceram. Soc. 53[6] (2016) 707-711.
-

- 18. H. Tian, H.-T. Liu, and H.-F. Cheng, Compo. Sci. Technol. 50[10] (2014) 202-208.
-

- 19. B. Wang, H. Li, L. Xu, J. Chen, and G. He, RSC Adv. 20[7] (2018) 12126-12132.
-

- 20. Y. Sasaki, Y. Nishina, M. Sato, and K. Okamura, J. Mater. Sci. 22[2] (1987) 443-448.
-

- 21. K. Morishita, T. Matsumoto, S. Ochiai, H. Okuda, T. Ishikawa, and M. Sato, Mater. Trans. 48[2] (2007) 111-116.
-

- 22. M. takeda, A. Urano, J.-I. Sakamoto, and Y. Imai, J. Nucl. Mater. 259-263 (1998) 1594-1599.
-

- 23. S. Cao, J. Wang, and H. Wang, Cryst. Eng. Comm. 18[20] (2016) 3674-3682.
-

- 24. D.G. Shin, Y.J. Lee, E.J. Jin, K.Y. Cho, Y.H. Kim, S.R. Kim, W.T. Kwon, J.S. Hong, and D.H. Riu, J. Ceram. Process. Res. 15[5] (2014) 298-304.
- 25.
 This Article
This Article
-
2019; 20(5): 563-569
Published on Oct 31, 2019
- 10.36410/jcpr.2019.20.5.563
- Received on Jul 22, 2019
- Revised on Aug 5, 2019
- Accepted on Aug 14, 2019
 Services
Services
- Abstract
introduction
experimental procedure
results and discussion
conclusion
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kwang Youn Cho b and Cheol Jin Kim a
-
aResearch Institute of Green Energy Convergence Technology, Gyeongsang National Univ., Jinju 52828, Korea
bCeramic Fiber & Composites Center, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Korea
Tel : +82-55-792-2710
Fax: +82-55-792-2710 - E-mail: kycho@kicet.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.