- Effects of rod-like BN on dielectric and mechanical properties of Si3N4 porous composite prepared via gel-casting method
M. Vaseghia, S. Baghshahib,*, M. Mashhadic and A. Nematid
aDepartment of Materials Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
bDepartment of Materials Engineering, Faculty of Engineering, Imam Khomeini International University, Qazvin, Iran
cFaculty of Materials and Manufacturing Engineering, Malek Ashtar University of Technology, Tehran, Iran
dDepartment of Materials Science and Engineering, Sharif University of Technology, Tehran, Iran
Gel-casting is an appropriate method for manufacturing porous parts with complicated forms, which has also been used in shaping Si3N4 bodies. In the present study, porous bulk Si3N4 samples were fabricated using gel-casting. To this aim Si3N4 slurries were prepared with solid loading of 35 vol%. To the slurry was added 1 and 3 wt% rod-like BN to enhance the mechanical properties. BN was selected to further reduce the dielectric constant and loss of the prepared porous Si3N4 samples. The findings suggested that employing rod-like BN increased the porosity from 38 % to 41 %, reducing the flexural strength from 77 MPa in the part without BN to 56 MPa in the part containing 3 wt% BN. On the other hand, dielectric properties reduced in the parts with BN compared with the part without BN.
Keywords: Si3N4 gel-casting, Rod-like BN, Porosity, Mechanical strength, Dielectric loss and constant
Because of its excellent properties [1, 2] Si3N4 is a truly magic material, which has found its way in parts working at high temperatures such as gas turbines and combustion engines. Common methods of shaping ceramics such as isostatic press or hot press methods cannot be used to form Si3N4 into complex shapes. On the other hand, gel-casting is one of the best techniques particularly when the aim is manufacturing porous parts. Increasing the porosity is an effective way to reduce dielectric constant and tanδ and this can be accomplished by gel-casting. However, increased porosity means reduced mechanical strength. Therefore, it is necessary to use a strengthening phase. In applications where low dielectric loss and constant is an advantage, it is possible to use material such as BN which has dielectric properties lower than the Si3N4 base. Yao et al. [3] manufactured the porous body of Si3N4 with Isobam (as both dispersing and gelling agent) reporting reduced flexural strength in using BN. Zhao et al. [4] manufactured a BNpn/Si3N4 composite using 10 vol% BN nanoparticles and as a result of using BN, achieved the dielectric loss and constant of 0.006 and 4.31 while the formation of rod-like β-Si3N4 grain led to the flexural strength of 198/9 MPa. Kovalciková et al. [5] used a mixture of the BN powders with two sizes (nanoparticles and micro particles) with micron sized Si3N4 to prepare composites.
They showed that increasing the BN content reduced the density of the samples, although the density of the samples containing a mixture of micro/nano particles was more than that of the samples containing a mixture of micro/micro particles, resulting in better mechanical properties. Wang et al. [6] observed decreased dielectric loss and constant after adding BN particles. Li et al. [7] recorded dielectric constant of 2.7-3.3 for porous Si3N4 ceramics with 40-50% porosity; however, the problem with these ceramics was reduced resistance against corrosion, which was solved by covering their surface with a dense layer of Si3N4 by CVD method. In their studies on the microstructure and mechanical properties of porous Si3N4-BN-SiO2 ceramics, Long et al. [8] observed that these ceramics have lower elastic module and higher strength and toughness compared with monolithic porous Si3N4 ceramics.
A problem with BN is its hydrophobic property, which makes it difficult to disperse in aqueous media for preparing slurry. Therefore, the rod-like BN as explained in our previous study [9] was synthesized and used in the manufacturing of the samples to make slurry. The purpose of the study in making rod-like BNs, which is unprecedented in producing Si3N4 ceramics, was exploiting their rod-like morphology to improve the mechanical properties, so that in addition to reducing the dielectric loss and constant, the mechanical properties are also strengthened. This is because manufacturing Si3N4 composites with BN has always been accompanied by reduced mechanical properties.
Materials
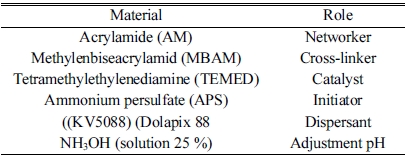
A monomeric system was employed for preparing the gel as shown in Table 1. Raw materials included α-Si3N4 powder (purity ≥ 95%; d50 = 1 μm, China) and rod-like h-BN (made in the laboratory based on reference.
Gel-casting preparation
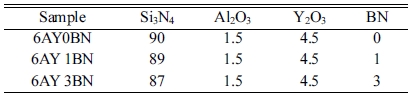
Since the Y2O3:Al2O3 sintering aid is a compound with low eutectic temperature as the related phase diagram shows, it can provide the system with the liquid phase at the temperature when the α-Si3N4→ β-Si3N4 begins to transform, which is about 1400 oC. Therefore, 94 wt% Si3N4 was initially mixed with 6 wt% sintering aid (3Y2O3:1Al2O3). In order to study the effects of BN on dielectric properties, 1 and 3 wt% rod-like BNs were added to the solid loading. The constituents of the samples are presented in Table 2. The slurry containing 50 wt.% solid loading was prepared by mixing the premix solution with 1 wt.% Dolapix 88 as dispersant and AM and MBAM monomers with the AM/MBAM ratio of 1:3 in distilled water.
Ceramic powders were then added to the premix solution and mixed to make the slurry, which was homogenized in a ball mill for 24 h. Next, the initiator and the catalyst were added and mixed and the slurry was immediately cast into a polyethylene mold. Gel samples were then removed from the mold and dried at ambient temperature and humidity for 48 h. The heat treatment was performed for burnout at the heating rate of 1 oC/min up to 600 oC and a soaking time of 4 h. Afterwards, the samples were heated at 1,650 oC for 2 h in order to sinter at the heating rate of 10 oC/min.
Bulk density of the sintered samples were measured using Archimedes’ principle and Equation (1):

where ρb, ρliq, md, mi, and ms are bulk density, the density of water under experimental condition, dry mass, submersion mass, and saturation mass of the samples, respectively. The percentage of open porosity (PO%) was also calculated using Equation (2):

Phases were identified using an X-ray diffraction device (XRD-3003PTS, SEIFERT) and a scanning electron microscope (SEM VEGA II) was used to analyze the samples’ microstructures. Three point flexural strength test was using testing equipment (Zwice Z1005) with the loading speed of 0.2 mm/min. Samples of 50 × 40 × 3 mm3 size were cut and polished for this purpose and the distance with the support was set at 40 mm. Flexural strength (σ) was calculated using Equation (3):

where P is the fracture force (N), L is the span length (mm), b is the specimen width (mm) and h is the specimen thickness (mm).
In order to evaluate the dielectric properties of the samples, dielectric constant and tanδ test (Agilent (Keysight) Technologies E8362) was carried out on 3 samples of 10 × 3 × 22 mm3 size for each batch.
Properties of the Rod-like BN
Fig. 1 depicts the Scanning Electron Microscope (SEM) image and X-Ray Diffraction (XRD). As the images show, the synthesized BNs are rod-like and with various sizes; the XRD pattern suggests the formation of BN crystals.
XRD phase and microstructure analysis of the sintered samples
Fig. 2 shows the XRD pattern of the porous composites. Specific peaks were observed for detecting the α-Si3N4, β-Si3N4 and Si2N2O phases.
Effects of rod-like BN on formation of final phases
As shown in Fig. 2, the α-Si3N4 peaks in 6AY0BN sample are less prominent than those in the samples containing BN rods; also, the peaks’ prominence in samples containing 1 and 3 wt% BN is increased. It is therefore evident that the main phase in the samples without BN is β-Si3N4 and adding rod-like BNs reduced the formation of β-Si3N4 phase and, in return, increased the intensity of Si2N2O peaks. Yao et al. [10] found that with an increase in BN content, the size of β-Si3N4 grains reduced and BN prevented transfer of the material during dissolution- sedimentation process. However, their XRD results suggested the occurrence of α-Si3N4 → β-Si3N4 transformation, where in all the samples, the main phase was β-Si3N4.
Wan et al. [11] evenby changing the sintering aid in the presence of 10 wt% H-BN particles reported a complete transformation and detected no α-Si3N4 phase. In their study, Zhao et al. [12] using BN abundant nanoparticles (5-20 vol%), confirmed XRD patterns of formation of β-Si3N4 phase and trans formation. Therefore, based on the findings of these studies, BN particles cannot account for the delay in transformation.
Effects of sintering aid on the phase formation
XRD patterns evident that β-Si3N4 and Si2N2O phases are formed under transformation phases. Like Si3N4, Si2N2O is formed in a liquid phase rich with SiO2 through a dissolution-penetration-sedimentation process. Dielectric loss and constant in Si2N2O is less than in Si3N4, which reduces the dielectric properties in the composite. The α particles dissolve in the liquid phase through penetration of N and Si atoms in the common face of the liquid phase/α and therefore, transformation occurs by breaking the bonds in the α particles and then development of the bonds in the form of singular β cells [13]. Therefore, the nucleation and growth of the secondary phase is dependent on the speed with which the α particles dissolve in the liquid phase. If in the interface between the liquid phase/α, O can penetrate into SiN4 tetrahedrons, it can stop α → β transformation. At the same time, if O can enter the Si3N4 unit cell, which is just formed, and can replace with one of the N atoms, it can generate Si2N2O phase unit cell by forming SiN3O tetrahedrons. In the sample without rod-like BNs, the amount of generated β-Si3N4 phase is more than that in the samples containing BNs and the intensity of the peaks in α-Si3N4 and Si2N2O phases is less. However, by adding rod-like BNs (in both samples containing 3 wt% and 1BN) the intensity of the peak in β-Si3N4 phase reduces while the intensity of the peak in Si2N2O increases. Therefore, it seems that since the amount of the liquid phase in these samples is low (6 wt%) and a relatively low temperature is used in the system, with the formation of Si2N2O in the 6AY1BN sample more liquid phase is consumed and in practice, less liquid phase was available for the system to form β and the penetration of oxygen into the structure of Si3N4 prevented the α → β transformation and at the same time resulted in the generation of more Si2N2O. On the other hand, in 6AY3BN sample, the increased intensity of the Si2N2O and β peaks was observed in comparison with the 6AY1BN sample. Thus, the amount of liquid phase can play a more effective role in formation of the final phases. Fig. 3 shows the substructures of 6AY0BN and 6AY1BN samples, which suggest transformation of more a particles and growth of Si2N2O and β phases after adding the BN.
Density and Porosity
Porosity is inevitable in the samples formed by gel-casting and then sintering. Also, porosity is necessary in order to reduce dielectric constant & tanδ. As SEM image in Fig. 4 shows, there are pores with relatively large diameters in the samples containing 1 wt% rod-like BN and samples without rod-like BN.
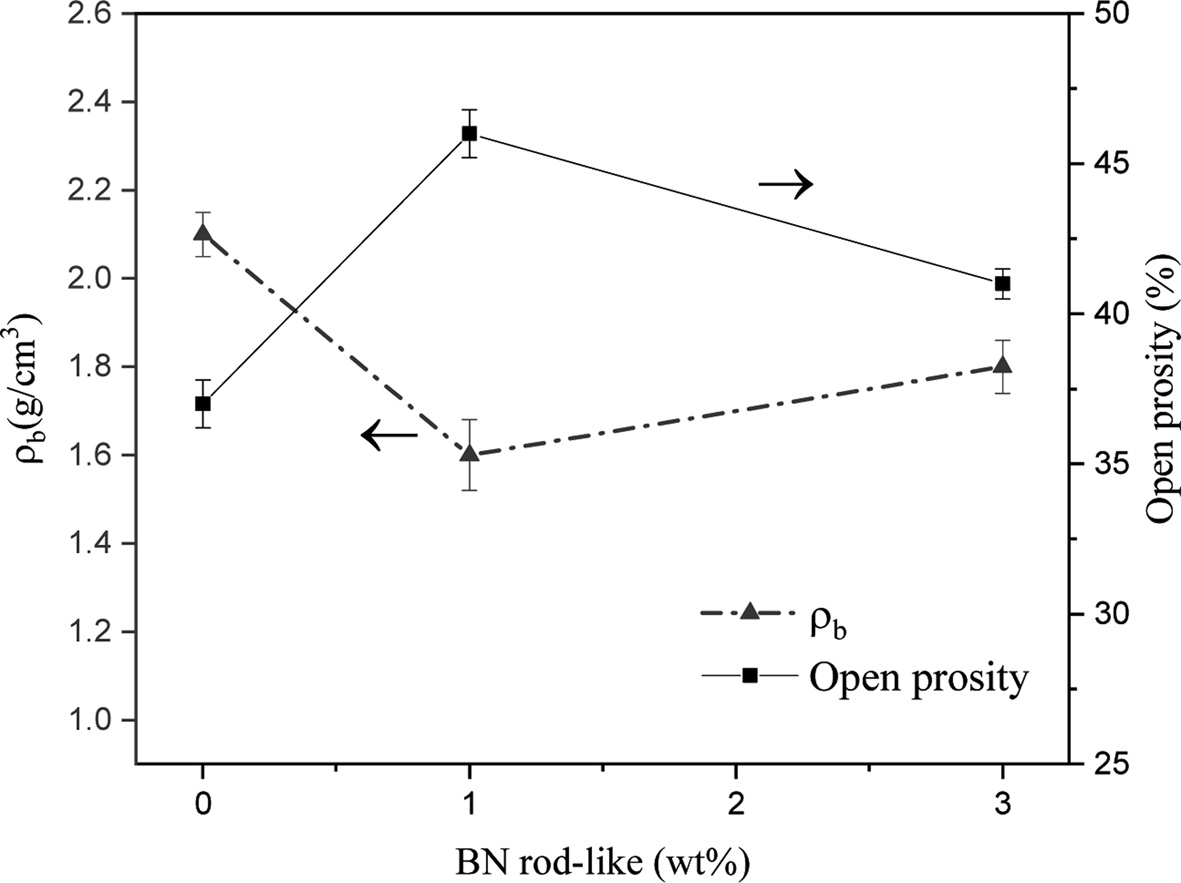
Results of the measurement of bulk density (ρb) and open porosity percentage (Po%) present in the samples are shown in Fig. 5. As shown, with an increase in the rod-like BN content, the porosity first increases and then shows a slight decrease. Therefore, it can be seen that the addition of BN resulted in porosity increase in specimens, which is evident from SEM images and density measurements.
Adding rod-like BN as a secondary phase, because of incompatibility of the properties of the background phase and the secondary phase, results in the increased porosity in the background. Therefore, adding BN coincides with increased porosity and reduced density of the samples. However, when the 1 wt% BN is added to the combination of the raw materials, the porosity increases and by adding 3 wt% BN, a slight decrease is observed in the porosity. As was explained in the section on phase analysis, since adding BN coincided with reduced Si3N4, the ratio of sintering aid to the main phase slightly increased after 3 wt% BN was added and probably resulted in a better rearrangement of the particles as its effects are observed in the slight reduction in the porosity compared with the samples containing 1 wt%. On the other hand, since Si2N2O has a lower density compared to Si3N4, after its formation in the composition of final phases, the density reduced.
Flexural strength
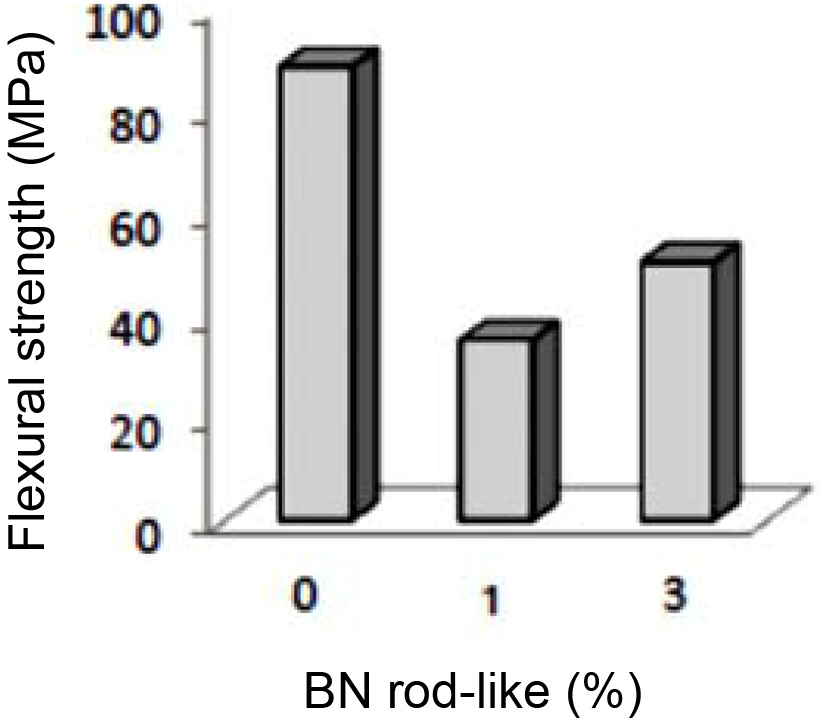
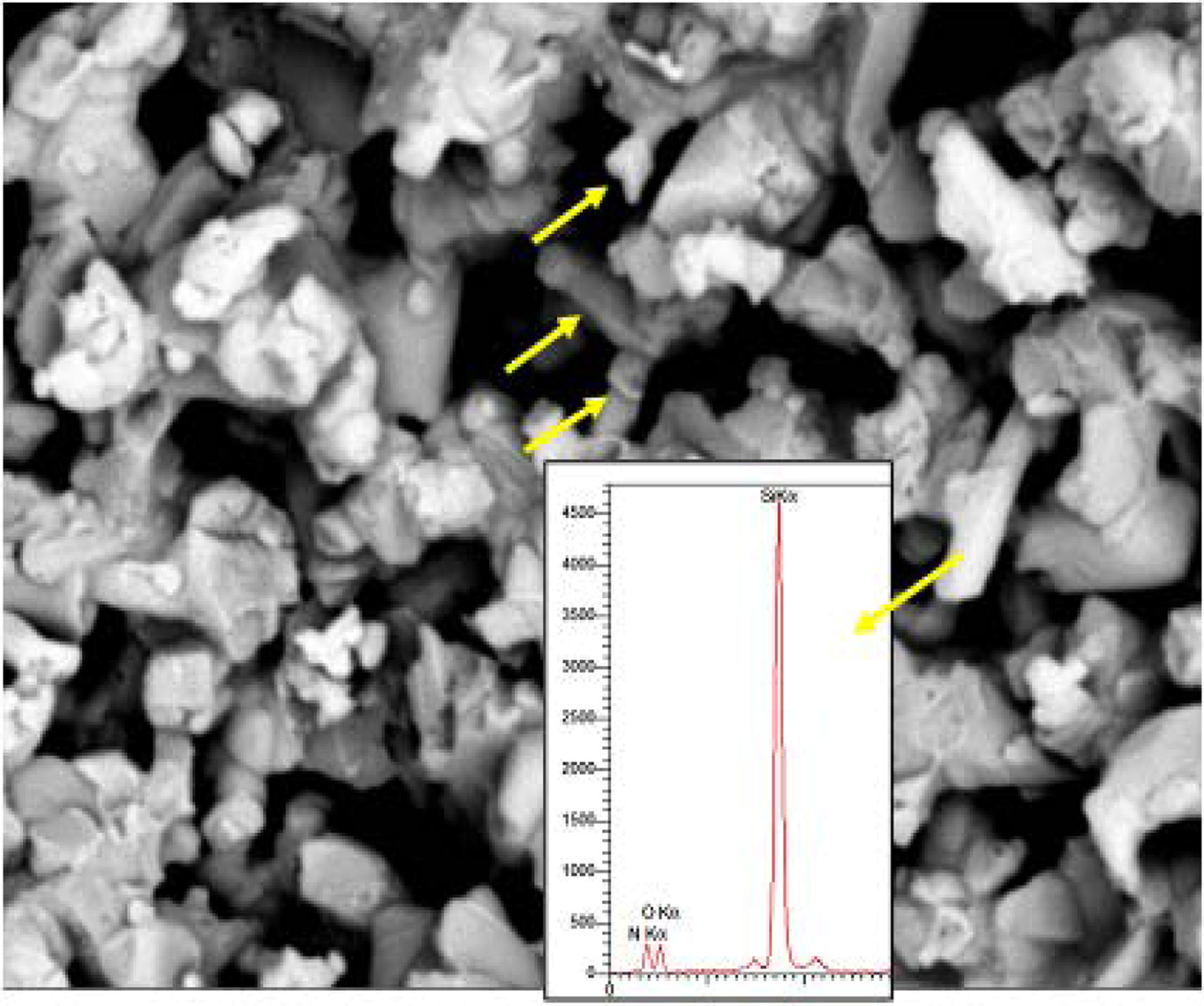
The result of three-point flexural strength tests on 6AY samples are shown in Fig. 6. Findings reveal that adding rod-like BN reduced the flexural strength. Porosity is the most important factor responsible for reducing flexural strength, therefore, if sintering fails to reduce porosity and create bonds among the grains, the pores can act as centers for propagation of cracks and reduce the flexural strength. Thus, in conditions where porosity needs to be present in the body, strengthening microstructure through developing an interwoven structure is a method of improving the mechanical properties of ceramics. The lowest flexural strength was observed in the sample containing 1 wt% BN, which was due to more porosity. On the other hand, when 3 wt% rod-like BN was added to the mixture, porosity reduced and this led to increased flexural strength in the sample 6AY3BN as compared to with the sample 6AY1BN. The SEM image of the rod-like grain of Si2N2O in the background of Si3N4 is shown in Fig. 7. As the figure suggests, the development of Si2N2O grains along with the presence of rod-like BN effectively improved the flexural strength in the sample with 3 wt% compared to 1 wt% BN.
Dielectric properties
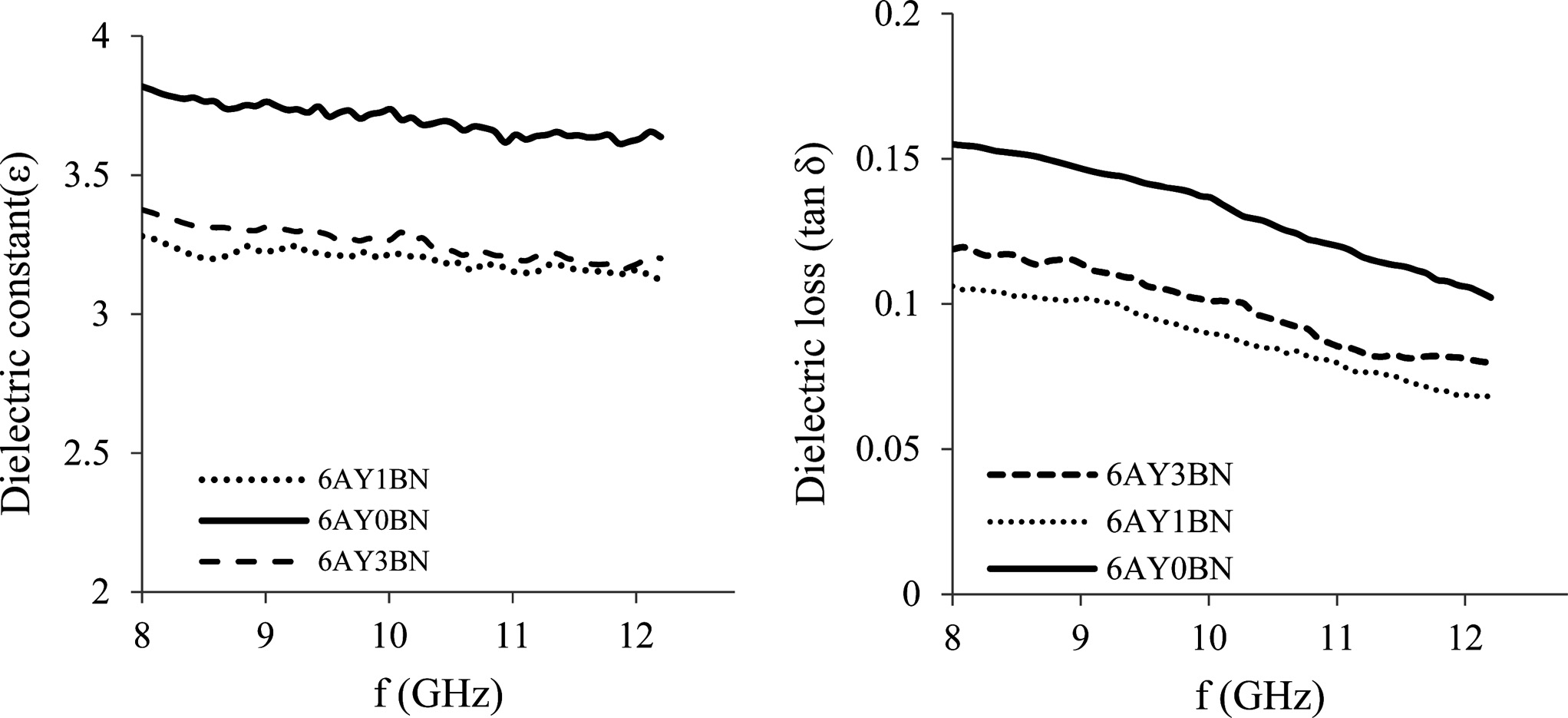
The dielectric loss and constant of the composites are shown in Fig. 8. The dielectric properties of the ceramic composites are largely dependent on the amount of porosity and the phase composition. Since α-Si3N4 and Si2N2O have lower dielectric loss and constant than β-Si3N4, the samples containing BN with more Si2N2O have lower dielectric loss and constant. Shuqin et al. [14] also observed that the formation of more Si2N2O phase resulted in a drop in the dielectric properties of the composites. On the other hand, in 6AY1BN and 6AY3BN samples, the presence of more pores and the formation of more α-Si3N4 and Si2N2O phases in the 6AY1BN
samples resulted in lower dielectric loss and constant compared with 6AY3BN samples.
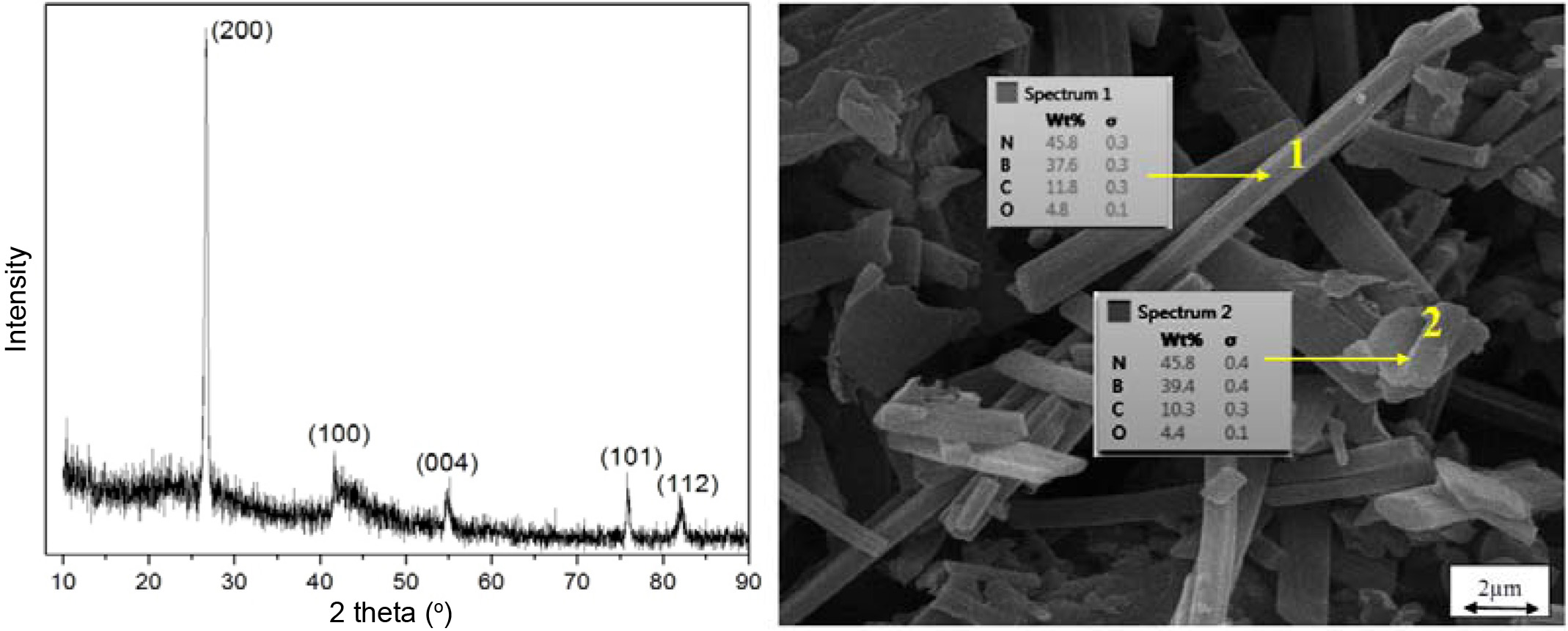
|
Fig. 1 XRD (left) and SEM image (right) of the rod-like BNs synthesized in the laboratory. |
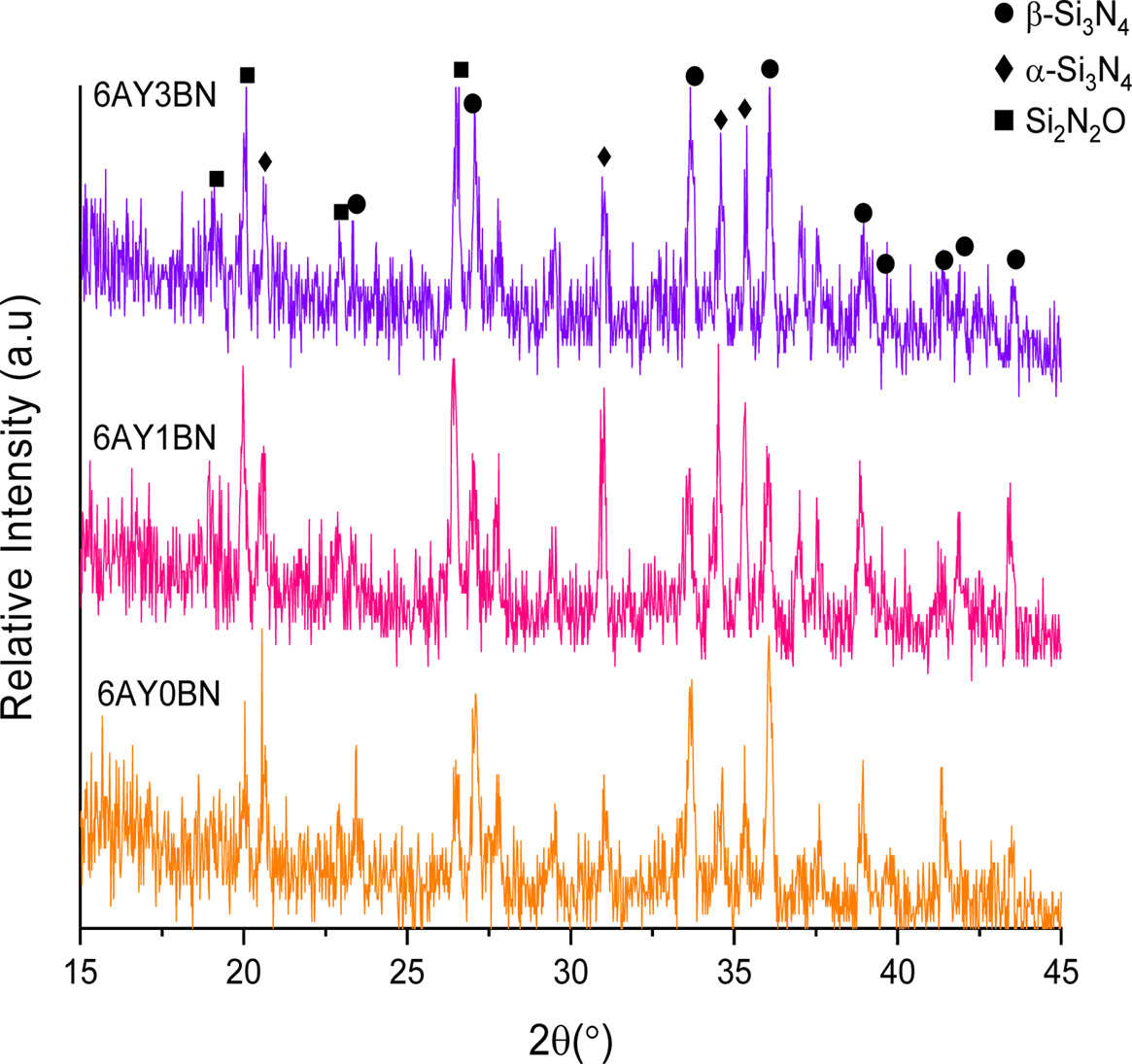
|
Fig. 2 XRD pattern: primary Samples containing 6 wt% sintering aid (Y2O3: Al2O3). |
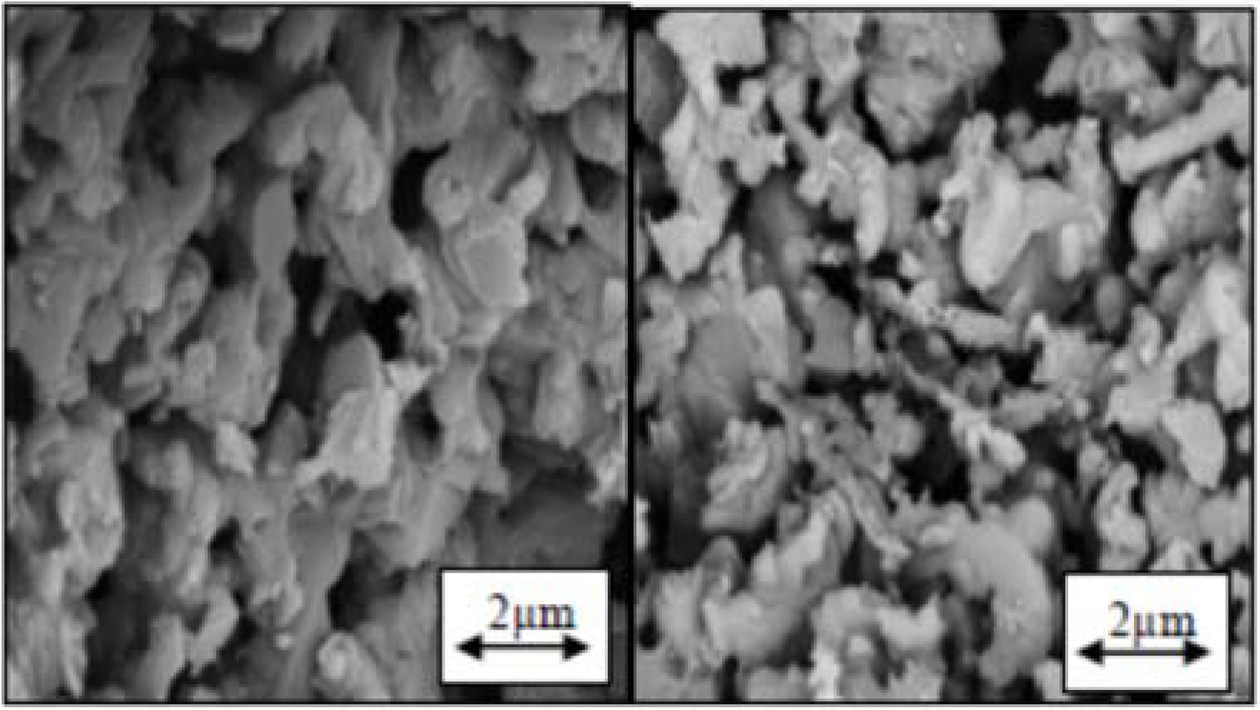
|
Fig. 3 SEM image; Left: 6AY0BN sample; right: 6AY1BN sample. |
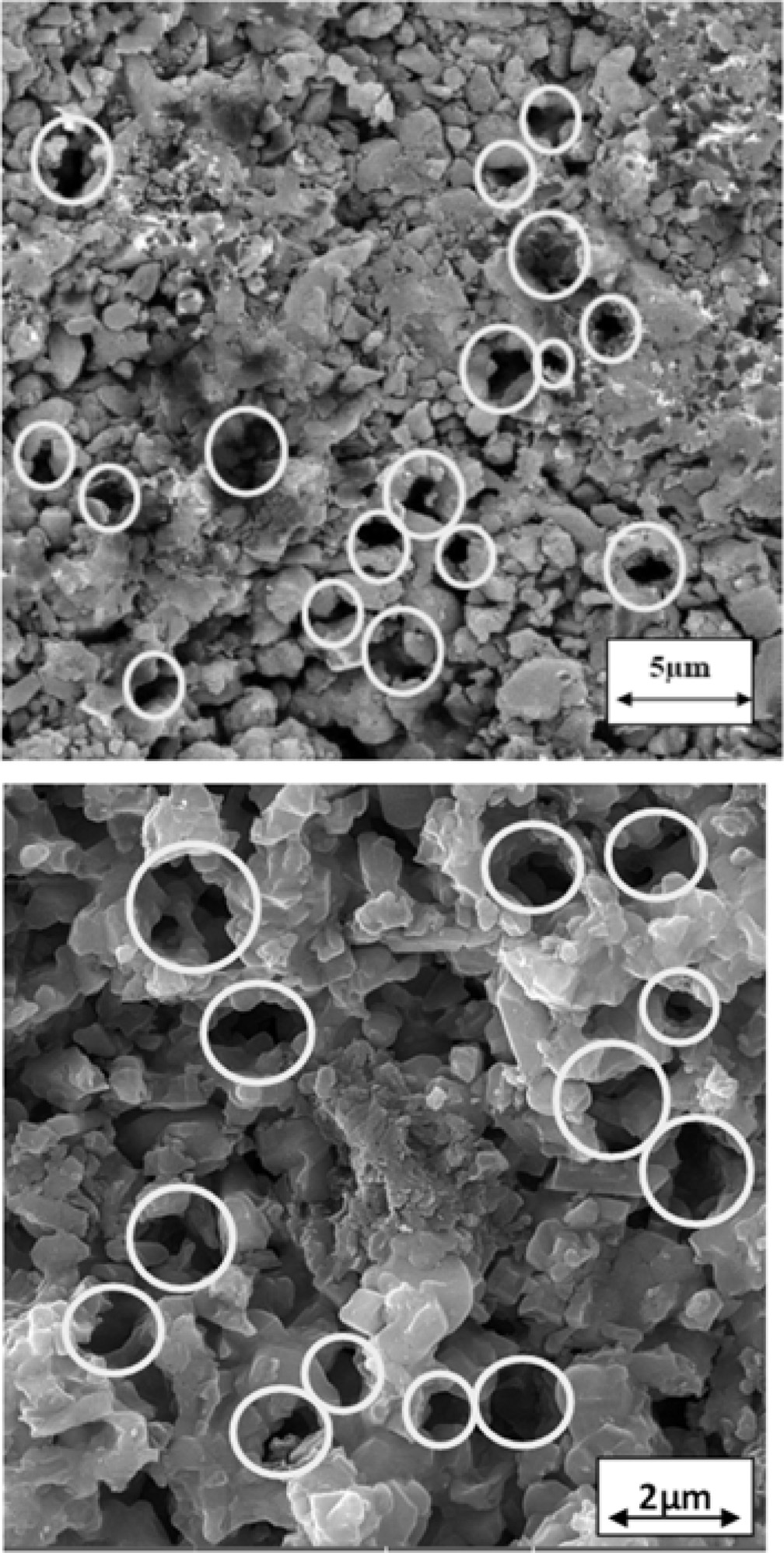
|
Fig. 4 SEM image of the porosity in the 6AY0BN (up) 6AY1BN (down). |
|
Fig. 5 Bulk density (ρb) and open porosity (Po%) of the samples containing 6 wt% Al2O3-Y2O3 sintering aid. |
|
Fig. 6 Flexural strength in samples 6AY based on wt% rod-like BN. |
|
Fig. 7 SEM image of the rod-like Si2N2O grains n the matrix of Si3N4. |
|
Fig. 8 Dielectric constant (left) and dielectric loss (right) of the 6AY samples based on frequency (GHz). |
In the porous bodies of Si3N4 manufactured by gel-casting, with an increase in the rod-like BN, the porosity increased in the samples. The strength of the samples containing BN was less than the sample without BN. Since increasing the BN meant reduced background Si3N4 powder, the liquid phase in the sintering temperature increased and more liquid phase in turn, improved the formation of β-Si3N4 and Si2N2O grains. Therefore, the presence of rod-like BN and rod-like Si2N2O grains increased the flexural strength of 6AY3BN as compared with 6AY1BN. Higher porosity and Si2N2O phase led to the lower dielectric loss and constant in the samples containing 1 wt% BN.
- 1. G. Ziegler, J. Heinrich, and G. Wotting, Journal of Material Science 22[9] (1987) 3041-3086.
-

- 2. A. Pyzik and D. Beaman, Journal of the American Ceramic Society 76[11] (1993) 2737-2744. doi.org/10.1111/j.1151-2916.1993.tb04010.x
-

- 3. T. Wan, D. Yao, J. Yin, Y. Xia, K. Zuo, and Y. Zeng, Internatinal Journal Application Ceramics Technology 12[5], (2015), 932-938.
-

- 4. D. Zhao, Y. Zhang, H. Gong, B. Zhu, and X. Zhang, Journal of Nanomaterials, doi:10.1155/2011/246847, (2011).
-

- 5. A. Kovalckov?, J. Balko, C. Bal?zsi, P. Hvizdo, and J. Dusza, Journal of the European Ceramic Society 34 (2014) 3319-3328.
-

- 6. S. Wang, D. Jia, Z. Yang, X. Duan, and Y. Zhou, Ceramics International 3 (2013) 4231-4237.
-

- 7. X. Li, X. Yin, L. Zhang, and T. Pan, International Journal of Applied Ceramic Technology 8[3] (2011) 627-636.
-

- 8. N. Long, J. Bi, W. Wang, M. Du, and Y. Bai, Ceramics International 38 (2012) 2381-2387.
-

- 9. M. Vaseghi, S. Baghshahib, M. Mashhadi, and A. Nemati, Journal of Ceramic Processing Research 20[2] (2019) 121-126.
- 10. D. Yao, Y.P. Zeng, K. H. Zuo, and D. Jiang, Journal of the American Ceramic Society 94[3] (2011) 666-670.
-

- 11. T. Wan, D. Yao, J. Yin, Y. Xia, K. Zuo, and Y. Zeng, International Journal of Applied Ceramic Technology 12[5] (2015) 932-938.
-

- 12. D. Zhao, Y. Zhang, H. Gong, B. Zhu, and X. Zhang, Journal of Nanomaterials (2011) doi:10.1155/2011/24684.
-

- 13. D. Bu?evac, S. Bo?kovi?, and B. Matovi?, Science of Sintering, 40 (2008) 263-270.
-

- 14. L. Shuqin, P. YuChen, Y. ChangQing, and L. JiaLu, Ceramics International 35 (2009) 1851-1854.
-

 This Article
This Article
-
2019; 20(5): 512-517
Published on Oct 31, 2019
- 10.36410/jcpr.2019.20.5.512
- Received on Mar 8, 2019
- Revised on Jun 2, 2019
- Accepted on Jun 3, 2019
 Services
Services
- Abstract
introduction
materials and methods
characterization
results and discussion
conclusion
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- S. Baghshahi
-
Department of Materials Engineering, Faculty of Engineering, Imam Khomeini International University, Qazvin, Iran
Tel : +98-9122164225
Fax: +98-2833780073 - E-mail: baghshahi@eng.ikiu.ac.ir






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.