- Efficiency of gerAa, tupA and ca transformation in Bacillus subtilis for self-healing of concrete cracks
Hanxing Hea, Gefei Lib, Jiantao Zhangb, Jinlong Zhanga, Mingyue Luoa, Wenkai Huc, Yamin Lina, Ziyu Dengc, Zhicheng Liud, Weizhao Chena,* and Xu Denga,*
aCollege of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518055, PR China
bSchool of Medicine, Shenzhen University, Shenzhen 518055, PR China
cInstitute for Advanced Study, Shenzhen University, Shenzhen 518055, PR China
dCollege of Computer Science & Software Engineering, Shenzhen University, Shenzhen 518055, PR China
A genetic approach was proposed to modify the characteristics of Bacillus subtilis strain WB800 (B. subtilis WB800) for self-healing of concrete cracks. Three genes, namely gerAa which encodes germination receptors activated by L-alanine, tupA which is responsible for the synthesis of teichuronopeptide, and ca which encodes carbonic anhydrase (CA) catalyzing the synthesis of carbonate ion, were separately transformed into WB800. To protect bacterial cells from being squeezed, microspheres were produced with microcrystal cellulose (MCC) before the introduction of bacteria into the specimens. The results showed that the modified B. subtilis expressing GerA achieved 39.9% of germination ratio compared to 17% by the original host cells. With the transformation of tupA, the modified strain demonstrated higher resistance to alkaline environments, tolerating pH as high as 11, while the original strain only tolerated pH 9. The modified strain expressing CA induced more calcium carbonate than the original cells. Energy dispersive spectroscopy (EDS) identified the produced precipitate to be calcite (CaCO3). Moreover, a mathematical model was developed to optimize the influential factors of calcium precipitation process. Finally, based on the above results, an effective self-healing of concrete crack was achieved. This study may provide a promising strategy to improve the efficiency of bacterial self-healing of concrete cracks.
Keywords: Bacterial spores, Gene modification, Self-healing, Concrete crack, Modeling
Concrete cracks are very detrimental to cementitious structures and rapidly shorten the life spans of such structures. In contrast to the traditional repair method which is expensive and ineffective, bacteria-based self-healing of concrete cracks has been demonstrated to be a novel solution to this problem [1]. Prior addition of chemical compounds in the cement can help seal cracks but only to a certain extent [2]. In a typical self-healing process, self-healing bacterial spores with nutrients under proper protective conditions are introduced into cementitious structures during concrete production. As the concrete cracks, bacterial spores are triggered to start germination and grow to a vegetative state. The cells utilize nutrients and convert carbon sources to carbonate, and the carbonate reacts with Ca2+ to form CaCO3 precipitate, thus sealing the cracks. Therefore, self-healing bacterial strains should exhibit three characteristics: sporulation/germination ability, tolerance to high pH and carbonation ability. Generally, self-healing bacteria were screened and isolated from natural environments in previous studies [3, 4]. From a cellular biological perspective, the three required characteristics mentioned above reflect three types of cellular activities: germination, which determines the quantity of cells when the self-healing process is initiated; pH regulation, which allows bacterial cells to adapt to the alkaline environment inside concrete structure; and carbonic anhydrase (CA) activity, which catalyzes the carbonation reaction, yielding carbonic ion (CO32-), which can react with Ca2+ to produce CaCO3 crystals. Apparently, these cellular activities can be regulated and further optimized at the molecular biological level. Therefore, three related endogenous genes were selected to target the germination capacity, resistance to alkaline environments and CA activity, namely, gerAa, tupA, and ca, respectively.
The gerA operon from Bacillus subtilis encodes spore’s germinant receptor for L-alanine. Overexpression of tricistronic gerA operon may markedly increase the rates of germination of B. subtilis spores with L-alanine, particularly at low L-alanine concentrations. Furthermore, a highly efficient PsspB promoter was linked with gerAa in gerA operon. PsspB exhibits greater efficiency than most endogenous promoters. This promoter is expressed at a high level during sporulation in a forespore-specific manner [5]. The efficiency and precision of this vector is critical because the proportion of germinating spores is crucial during the self-healing process.
The tupA gene was cloned from Bacillus strain C-125. This gene encodes teichuronopeptide (TUP), which is a copolymer consisting of polyglutamic acid (PGlu) and polyglucuronic acid (PGlcU). TUP is one of the major structural components of the cell wall of Bacillus lentus C-125. The highly acidic derivatives of PGlu and PGlcU facilitate resistance to alkaline environments [6]. This design tries to enhance the resistance of cells to highly alkaline environment inside concrete structure.
The ca gene was cloned from Bacillus halodurans TSLV1. This gene encodes CA, which catalyzes the hydration of carbon dioxide (CO2) with higher efficiency than the endogenous enzymes and has little negative effect on the metabolic pathway of B. subtilis [7]. The capacity of CA determines how much carbonate ion will be produced in limited time. The produced carbonate ion binds with free calcium ion, yielding calcium precipitation.
As these three genes are closely connected to the key influential factors on microbial induced self-healing process, our work, therefore, aimed to evaluate the effects of these genes by transforming them into a suitable host stain separately. Meanwhile, in order to protect self-healing bacterial spores as introducing the spores into concrete specimen during the casting process, a microsphere embedding method was applied. The microspheres were designed to include nutrients and germinants (L-alanine in this study). The provision of support and protection by the microspheres, preventing direct contact between spores and concrete [8], are also of great importance. Therefore, the microspheres were composed of microcrystal cellulose (MCC), which prevents deterioration due to erosion. Furthermore, a regression model was built to measure the weights of different factors during the self-healing process for optimization. This study may provide an alternative strategy to develop a highly efficient and practical bacteria-based self-healing process of concrete cracks.
Strain WB800 is a modified B. subtilis strain stored in our laboratory. Genes associated with eight extracellular proteases are knocked out to prevent the degradation of extracellular products.
Three target genes, gerAa, tupA and ca, cloned from B. subtilis, Bacillus lentus C-125 and Bacillus halodurans TSLV1 respectively were employed in this study to construct recombinant strains. To construct vector PsspB-gerAa, plasmid pP43NMK which contained replicon of kanamycin resistance marker was digested with the restriction enzymes Hind III and Bam HI, and then the resultant fragment containing gerAa with strong promotor PsspB was inserted and ligated between the Hind III and Bam HI restriction sites (Fig. 1a). Same method was applied to construct vector P43-tupA-T1 and vector P43-CA-T1 except that restriction enzymes Kpnl and Hind III were used (Fig. 1b and 1c).
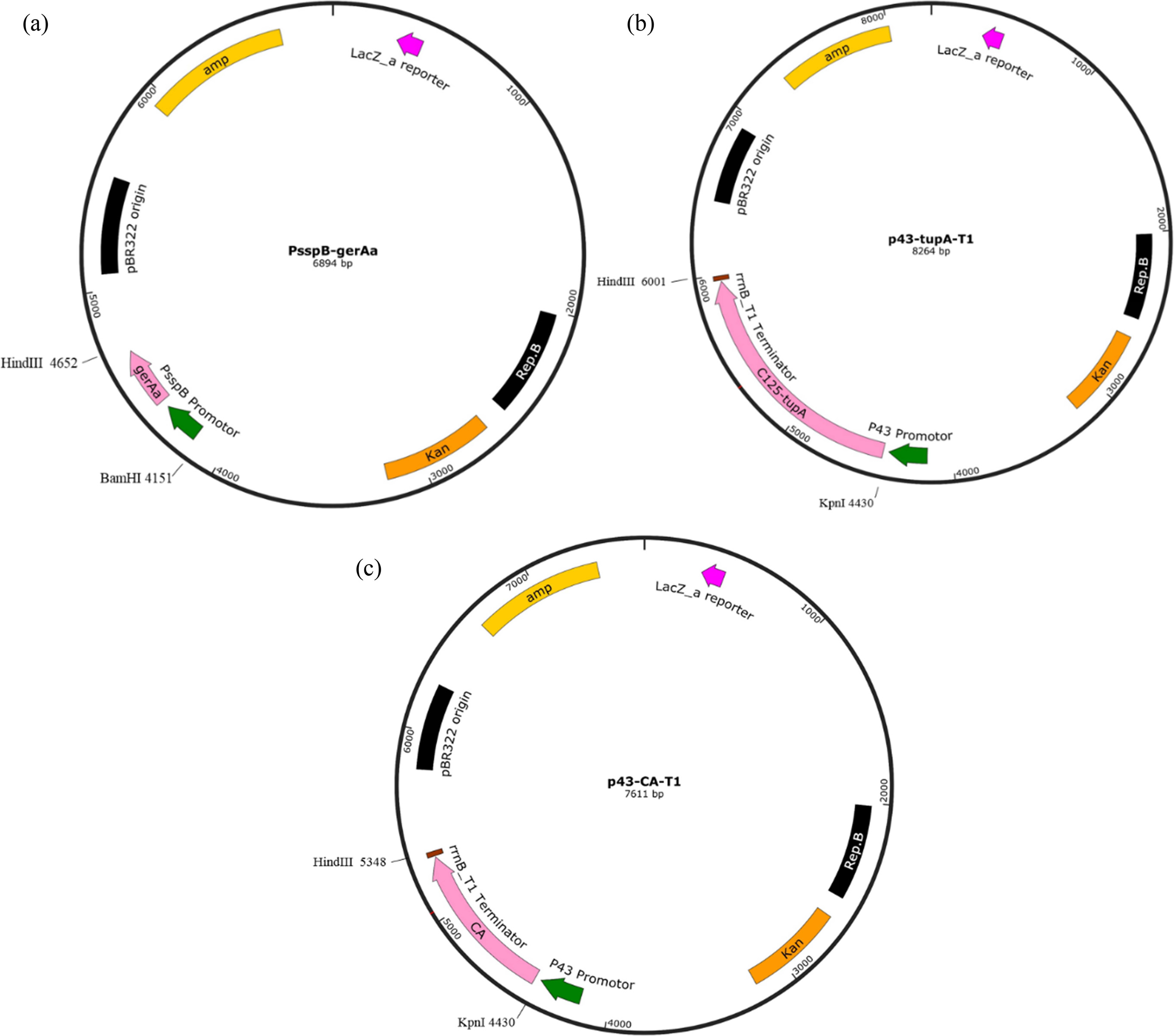
|
Fig. 1 Construction of recombinant plasmid. (a) plasmid containing gerAa, (b) plasmid containing tupA, (c) plasmid containing ca. |
The transformation of gerAa, tupA, and CA was performed by Spizizen method [9]. A single colony was used to inoculate liquid LB medium (Luria-Bertani medium) for propagation. 160 μL solution was extracted from LB medium and cultured in 8 mL of GM-I medium at 250 r/min for 4-5 h at 37 ℃. Then, 0.2 mL solution was extracted and cultured in 2 ml SP-II medium at 100 r/min for 90 min at 37 ℃. Then, 20 μl 10 mmol/L EGTA (Ethylene glycol-bis-N,N,N’,N’-tetraacetic acid) was added to the SP-II medium and incubated for 10 min [9]. The solution was incubated with shaking at 250 r/min for 90 min at 37 ℃ before cultivation in selective medium.
The positive clones were harvested by screening for kanamycin resistance and then cultivated in liquid LB medium for 12 h. The plasmids were extracted and subjected to restriction endonuclease digestion. SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) was performed to determine CA protein expression. All chemicals were purchased from Sigma Co. Ltd. All data are collected from 3 replication for each treatment.
Recombinant WB800 was harvested from selective medium and plated on 2 × SG medium [10] at 37 ℃ for sporulation. Spores were resuspended in phosphate buffer solution, and the absorbance at 600 nm (OD600) was monitored until the value of 0.8-1 was attained. Germination was monitored by recording the OD600 in a 96-well microplate reader (Tecan Infinite M200) after the addition of L-alanine-NaCl mixed solution [10].
Recombinant colonies were cultured at 37 ℃ in a gradient series of alkaline media (LB solid medium containing Na2CO3) and necessary nutrients (from pH 9 to 11). After 36 h, differences in growth were observed.
The calcium precipitation capability of the recombinant strain was evaluated as described previously [11]). EDS was performed to characterize the precipitate.
For a self-healing process of concrete crack, bacterial spores need to be introduced into concrete structure during manufacturing period. However, shearing force of mixing and stirring and shrinking of inner pores caused by concrete carbonization may harm spores badly. Therefore, we embedded spores into microspheres to provide protection before introducing them into concrete. MCC and clay were employed in our experiment to serve as microsphere material because of water insolubility, chemical stability and biological compatibility.
CHL nutrient solution (50 mL: 25 g sodium lactate, 5 g sodium nitrate, 0.0075 g potassium dihydrogen phosphate, 0.375 g magnesium sulfate, and 1.25 g inosine dissolved in sodium lactate) was used for dilution of spores. One gram of MCC and 0.1 g clay with 1.5 mL diluted spore-CHL solution was used to produce microspheres by rolling the mixture by hand. Replication of microsphere was produced to repeat concrete specimen experiments.
A microsphere containing recombinant spores was added into the specimen before concretion. The specimen was split from the ditch on each side, exposing the microsphere in each fragment of the specimen. The self-healing assay was established by sticking two parts of the specimen together and immersing the parts in the water.
The results are illustrated as the mean values and standard deviations. Statistical comparison was performed by applying a one-way analysis of variance. GraphPad Prism5 and MATLAB were used to analyze the data.
As shown in Fig. 2(a), gerAa linking to sspB promoter can be separated by agarose electrophoresis after digestion. The band corresponding to pP43NMK plasmid is located at approximately 7000 bp, and that of gerAa with the sspB promoter is located at approximately 550 bp. The band of tupA is located at approximately 1800 bp, and that of ca is located at approximately 1000 bp (Fig. 2b and 2c). As shown in Fig. 2(d), the recombinant strain (lane 1) exhibits a clear band at approximately 35 kDa in the SDS-PAGE gel, while the positive treatment (lane 2) exhibits the same band. The control treatment did not exhibit this band.
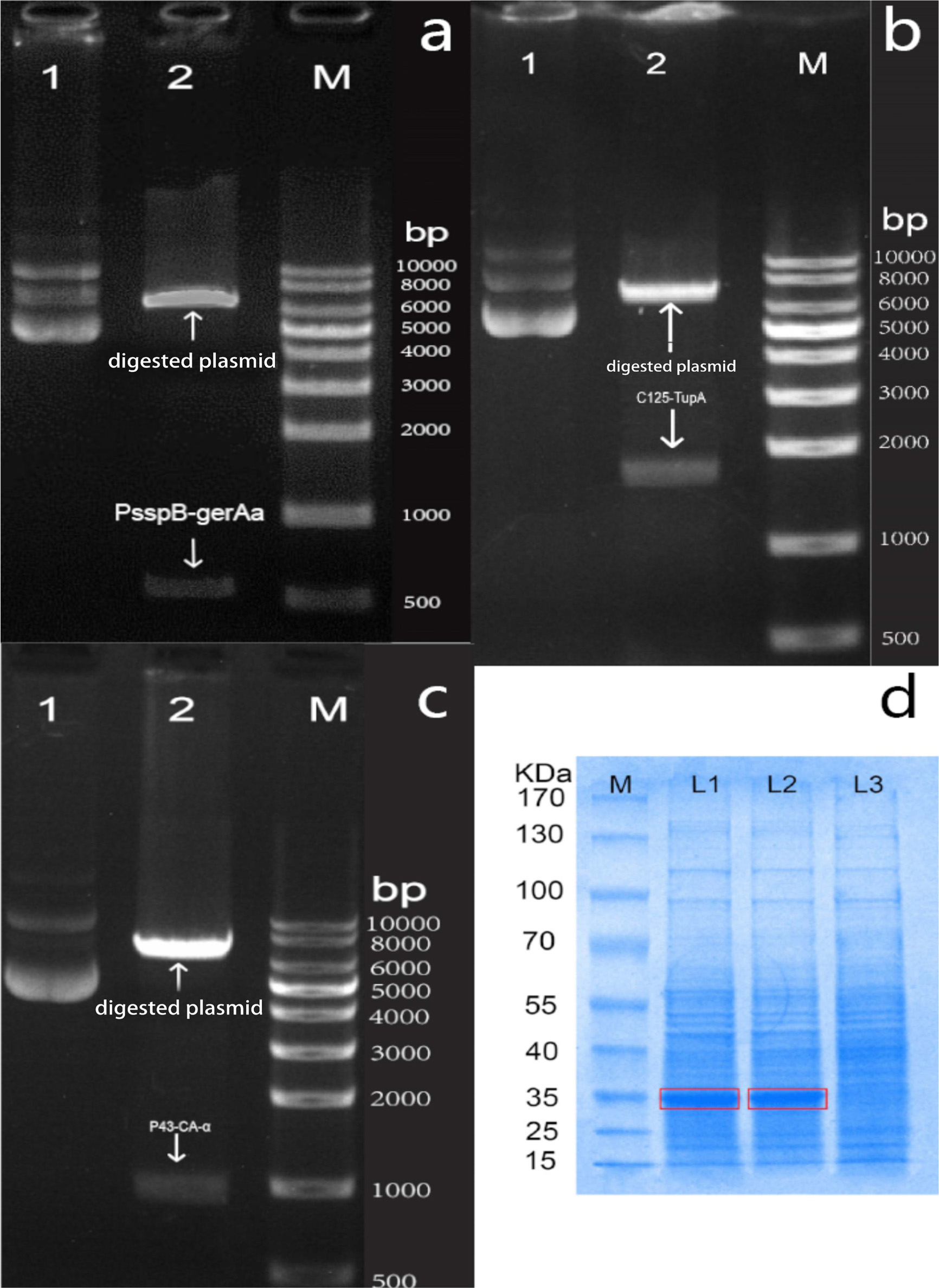
|
Fig. 2 Verification of plasmid transformation after digestion. (a) gerAa, (b) tupA, (c) ca, (d) SDS-PAGE analysis of the endocellular CA |
OD600 represents germination ratio, as this value generally decreases when spores germinate. In this experiment, both the control and recombinant treatments were treated with 4 mmol/L L-alanine simultaneously and incubated at 37 ℃ for 2 h.
As shown in Fig. 3, after 120 min of incubation, OD600 of the recombinant strain decreases from 1.1 to around 0.67, while that of the control only drops from 1.0 to 0.85. The reduction of absorbance of the recombinant strain is 39.9%, more than two times of 17% observed for the control, suggesting higher germination efficiency of the recombinant strain.
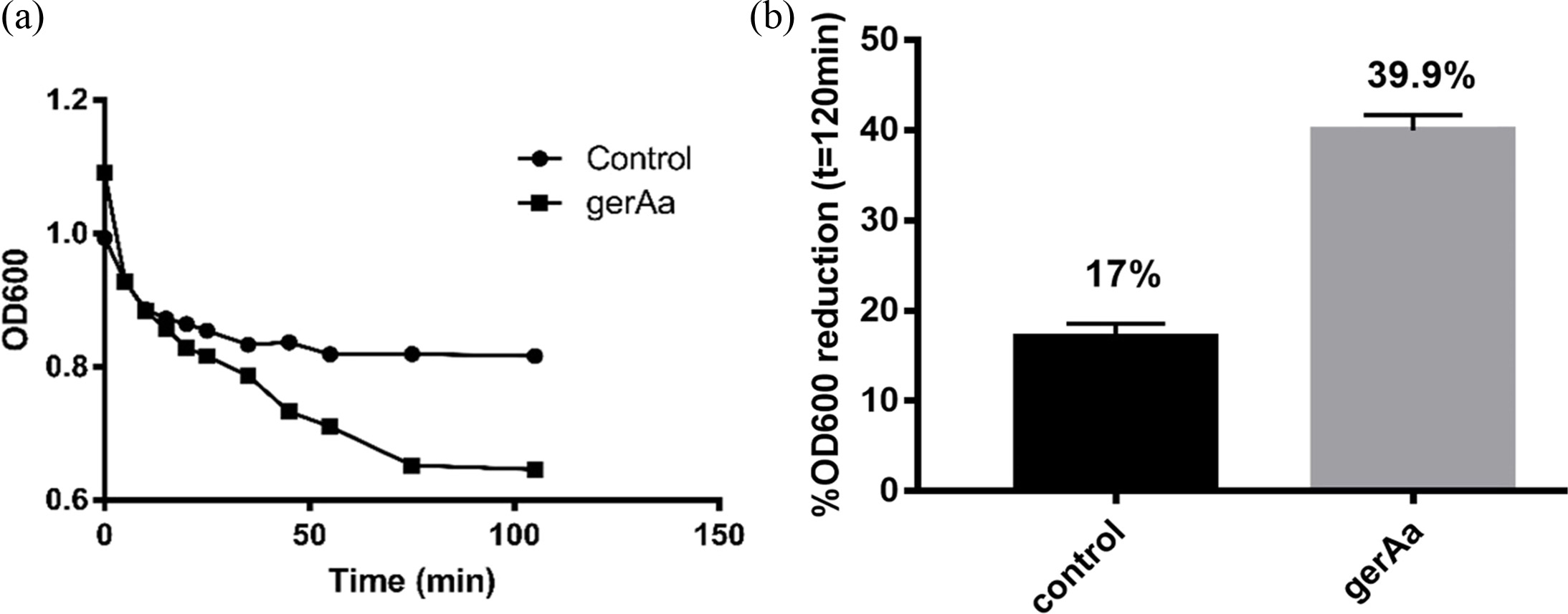
|
Fig. 3 Germination of bacterial spores. (a) Time course of OD600, (b) comparison of the reduction of OD600. |
The resistance of recombinant strain and original wild type to alkaline environment was evaluated and the results are shown in Fig. 4. It can be noted that the recombinant B. subtilis expressing TupA can grow on the plate in the range of pH from 9 to 11, whereas the original wild type can only form colony on the plate with pH being 9. When pH is up to 10, no trace of growth of wild type cells is observed.
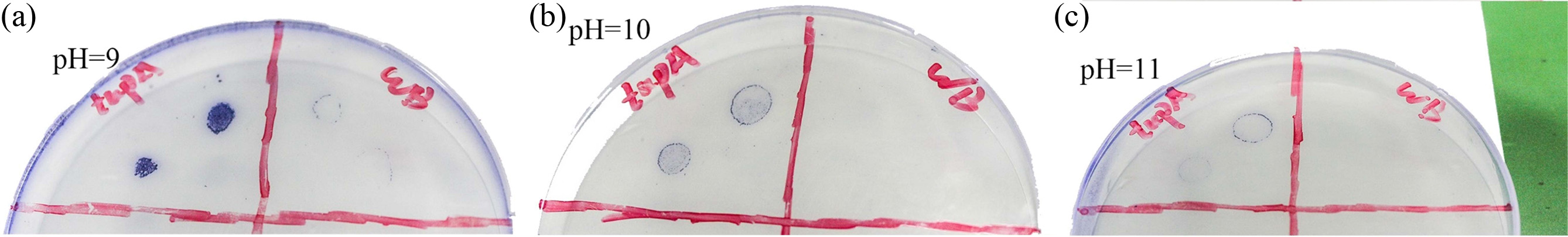
|
Fig. 4 Alkali resistance assay (The left part of each plate is cultured with recombinant B. subtilis, and the right part is the original host strain.). |
Precipitates was observed in the incubation system by the recombinant WB800 cells expressing CA. Energy dispersive spectrometry (EDS) analysis disclosed that the formed precipitates is primarily composed of calcium, carbon and oxygen, with a weight ratio closely matching that of CaCO3 (Fig. 5). The same substance was also harvested from the control treatment, but the weight obtained was much lower than that obtained by the recombinant strain for the same duration of precipitation.
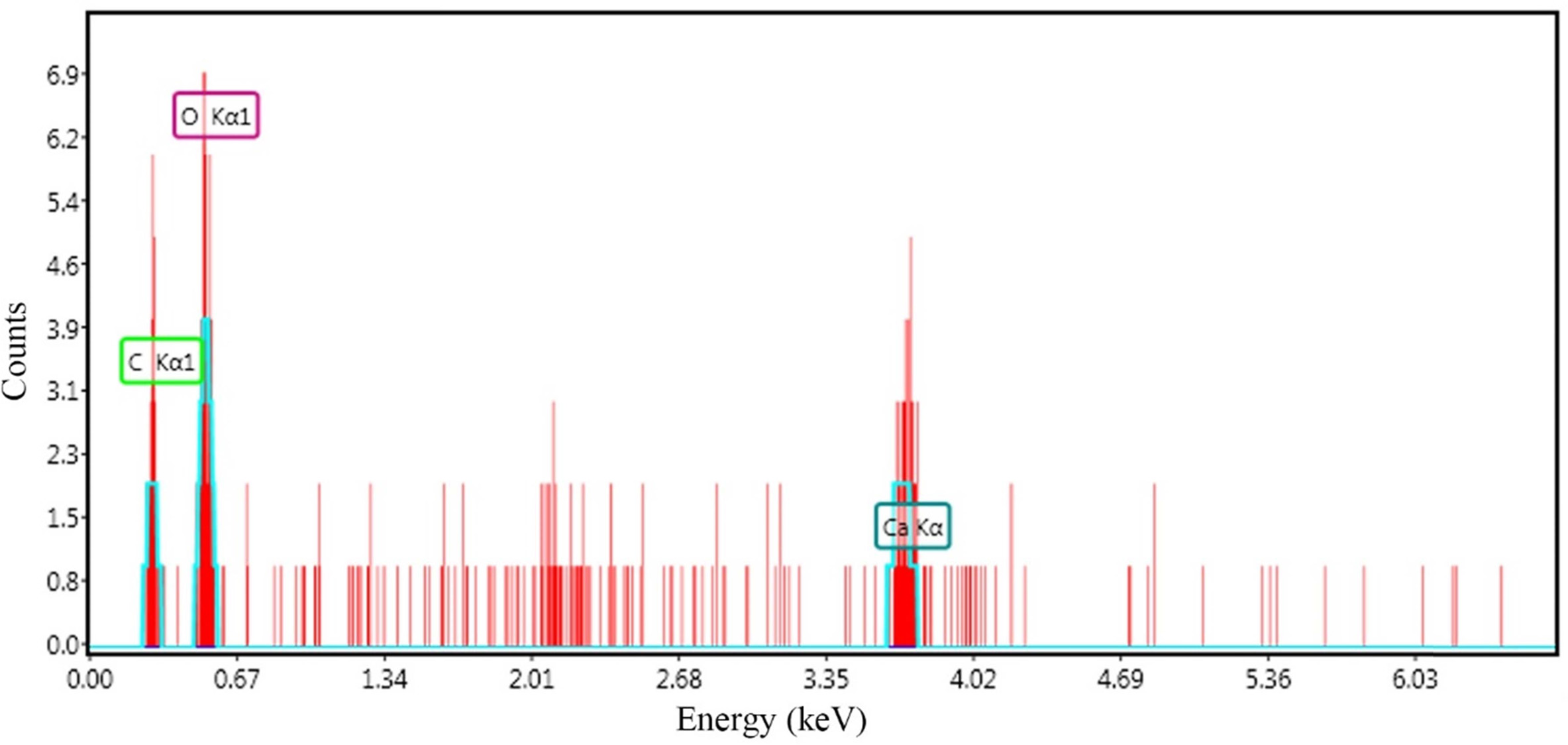
|
Fig. 5 Energy dispersive spectrum (EDS) analysis of the precipitate. |
Fig. 6(a) displays microspheres embedding spores with MCC and clay. Fig. 6(b) shows the microsphere that has been introduced into concrete specimen. Upon the occurrence of crack, the microsphere was split. Via scanning microscopy, it is clear that the concrete specimen under different treatments exhibits different repair results (Fig. 6c-e). After 12 days of incubation, the control specimen with no bacteria introduced shows no evidence of repairing (Fig. 6c), while in the presence of wild type cells, the crack of the specimen is partly repaired and only a few crystals are observed (Fig. 6d). However, a lot of precipitates were formed by the recombinant B. subtilis on the surface of the crack, consequently bringing about a complete crack healing (Fig. 6e).
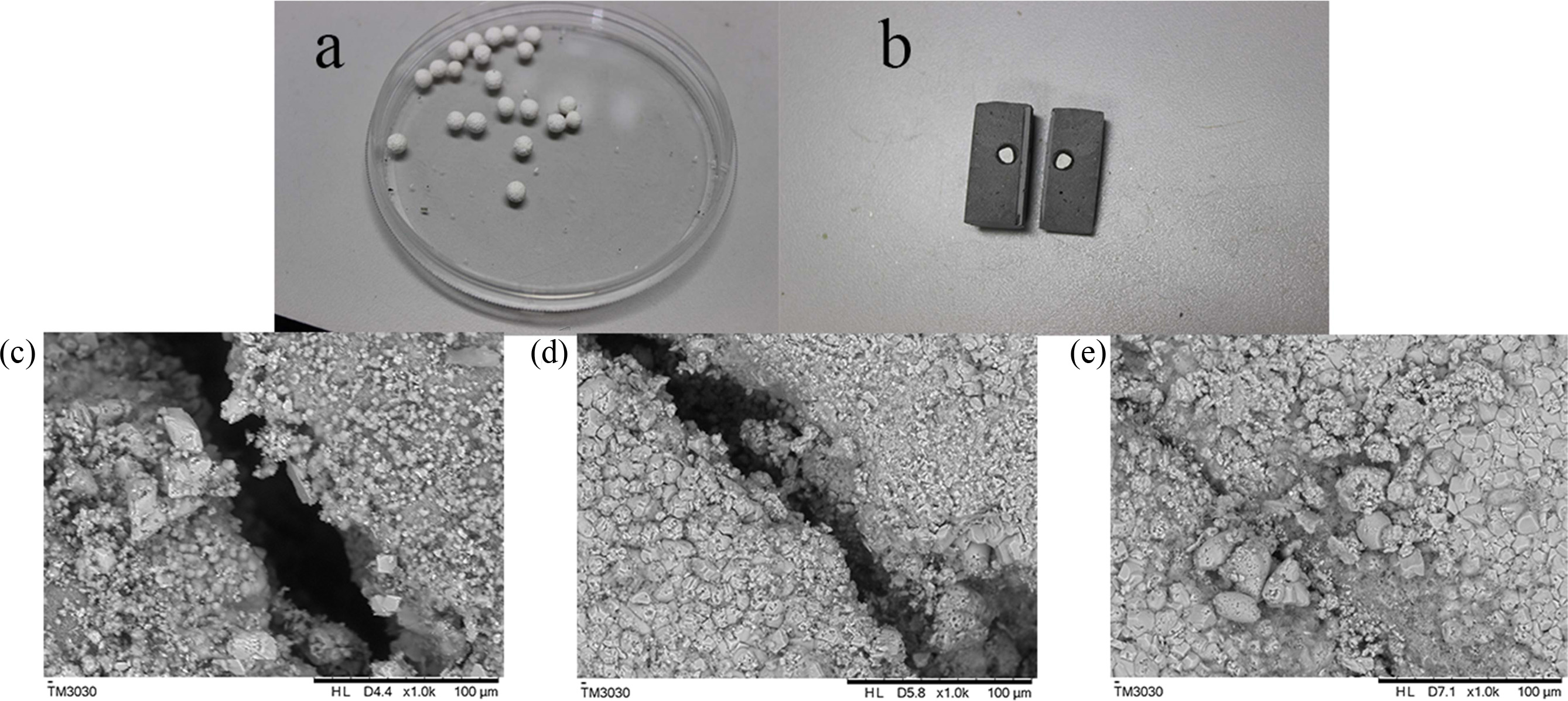
|
Fig. 6 Self-healing of concrete crack by bacterial cells. (a) Microspheres containing bacterial spores, (b) microsphere in concrete specimen. |
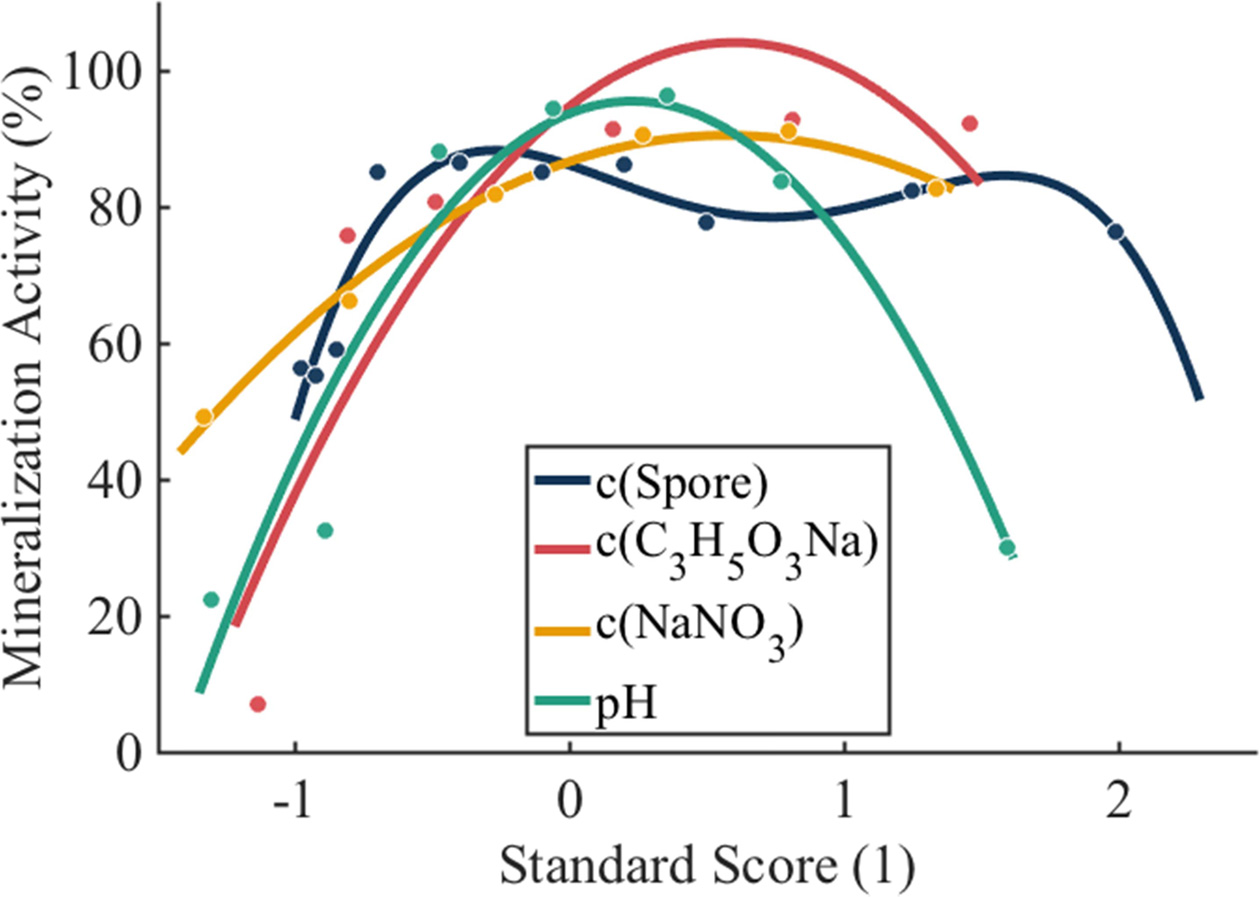
Optimization of the microspheres involved carbon source, nitrogen source, spore concentration and pH. A mathematical model was constructed to facilitate the optimization process. Mineralization activity was compared with standardized variables in the model. The Z-score standardization method was utilized as follows:
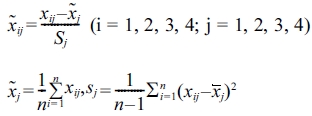
where 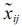 is the standard score, xij is variable,
is the standard score, xij is variable, 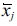 is the sample mean and Sj is the standard deviation.
is the sample mean and Sj is the standard deviation.
To confirm the relationship between the mineralization activity and each variable, MATLAB was adopted to accomplish curve-fitting for each single point, and a polynomial fitting method was adopted to obtain the equations below.
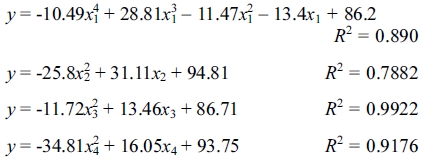
where x1 represents the carbon source, x2 represents the nitrogen source, x3 represents the spore concentration and x4 represents the pH (Fig. 7).
From the result in Fig. 7, a linear square method was used to obtain the overall relation between the four main variables and the mineralization activity as shown below.
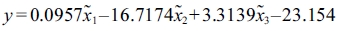
where 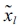 represents the curve-fitting equations in which the highest power term has a unit coefficient of 1.
represents the curve-fitting equations in which the highest power term has a unit coefficient of 1.
In the equation above, the nitrogen source has the maximum weightage, indicating that the nitrogen source is the most essential nutrient for B. subtilis. Additionally, the low weightage of the pH indicates that the spores are not sensitive to the changes in pH within a relatively appropriate range, but there is a sharp decline in activity when the pH reaches 11.
|
Fig. 7 Polynomial regression of influential factors. |
The microbial-based self-healing of concrete is strictly dependent on high alkalinity and some basic factors associated with spore growth (oxygen, humidity, and nutrient levels) [12]. The screening of suitable strains for self-healing concrete from natural environments might be technically difficult and ineffective. Therefore, the genetic approach provides a relatively practical and simple alternative. Given the shared expression system in prokaryotes, the efficiency of genetic modifications can be dynamic and varied. In this experiment, B. subtilis showed sufficient potential to be modified as designed.
The mineralization capability is dispensable for self-healing bacteria. Considering the fact that the inner porous environment of concrete is highly alkaline [13], bacteria used for healing of concrete crack must be alkaliphilic. Besides, for a self-healing process of concrete crack, bacteria need to be introduced into concrete structure during manufacturing period in the form of dormant spores. Upon the occurrence of crack, the ingress of water and air awakes spores and thus triggers the self-healing process [14]. Therefore, attempt to improve the resistance to pH and the efficiency of germination and mineralization of self-healing bacteria should be beneficial for the self-healing process of concrete crack [15, 16]. Our experimental results in Fig. 3-5 demonstrate that by means of transforming related functional genes, the recombinant strains respectively exhibit higher germination ratio, resistance to pH and mineralization ability than the control. Apparently our results suggest genetic approach is an effective alternative to improve the efficiency of self-healing of concrete crack. To our knowledge, no similar work was carried out in the previous study.
The concrete specimen was designed to simulate a possible situation in which the recombinant bacteria in the cracks were originally exposed to the external environment [17, 18]. In this study, a new concrete specimen was adopted, with the recombinant strain encapsulated in microspheres. From the result in Fig. 6, it can be noted that after introducing into concrete, the recombinant bacteria shows a more effective crack repairing than the wild type.
Although there is an effective approach in which cementitious material containing bacteria is used in self-healing concrete [19], MCC have proven to be more functional at sustaining the germination ratio and acting as a ‘switch’ for the regulation of gerAa. Total sealing of 1-mm cracks embedded with B. subtilis has been previously observed to occur within 28 days, which proved that endogenous carbonate secretion by this strain had been successful [20]. A similar result was obtained in this study in less than 28 days, indicating the enhancement of CA activity and secretion of carbonate due to the absence of the extracellular enzyme that degrades carbonate. In addition, a high CaCO3 yield can be obtained by using amine-modified magnetic iron oxide nanoparticles as carriers of bacteria [21, 22]. Furthermore, compared to other organic self-healing materials such as epoxy and PVA [23], CaCO3 induced by bacteria is not only chemically stable, but also totally inorganic and highly compatible with cementitious matrix. Therefore, microbial induced CaCO3 is a kind of suitable self-healing materials for concrete crack.
Blank treatment with no microspheres embedded in the concrete is necessary to account for crystals obtained due to the CO2 dissolved in the water [24]. Moreover, microsphere is necessary as a protection carrier to ensure an effective self-healing process [25].
In addition, decreased water penetration was also observed in our experiment (data not shown), which could be attributable to the blocking caused by bacterial induced precipitates [26]. Further investigation is necessary to determine the structural strength and durability of the concrete [27].
In order to realize an effective self-healing process, it is necessary to optimize the influential factors. In Amiri and Bundur’s work, when corn-steep liquor was used as a new carbon source, they developed a mathematical model to optimize the supplementary and alternative sources of other components based on the effect of calcium carbonate precipitation [28]. On the other hand, large-scale tests of self-healing concrete can be used in combination with mathematical model for optimization of the distribution of the polymeric components [29]. In addition, model reprogramming with a sensor can be used to monitor changes among factors over time, which is recommended in future applications [30].
The different coefficients are specific for various types of media, and the combination of conditions may require a flexible strategy [31]. However, the regression model used in this study was based on LB medium, which contains ions that are involved in self-healing of cracks. The composition of concrete was also excluded from the factors in the regression model. It is necessary to establish a model that considers predetermined environmental factors in addition to nutrients and spore concentration [3]. Currently, there are some factors such as viscosity that cannot be quantified due to the limitations of sensing methods [32].
To determine the probability of cracks being exposed with embedded microspheres, the microsphere-based method can be adopted with the correction of scale. The probability that at least one unit (microsphere) is exposed to crack can be derived as follows:
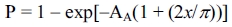
where AA denotes the area fraction of the microsphere, and x represents the crack-to-microsphere size ratio [33].
Furthermore, inside concrete structure, oxygen levels decrease with the increasing depth. Oxygen plays an important role in germination of self-healing strains [34]. In Qian’s experiment, a simulation of constant airflow above water containing cement was investigated to verify the occurrence of CO2 consumption during the bacteria-based self-healing process, wherein efflorescence imaging was used to quantitatively measure the repair status on the surface [35]. Presumably, the transformation from image statistics to regression function provides predictions and the coefficients for this reaction.
Recombinant B. subtilis WB800 expressing GerAa, TupA and CA shows higher spore germination ratio, higher resistance to pH and higher mineralization ability compared to the original host strain. After introducing into concrete specimen, the recombinant cells protected by microsphere can effectively repair concrete crack. Furthermore, a mathematical model was developed to optimize the influential factors of self-healing process and found nitrogen source is the most important factor. This study may provide an effective alternative strategy to improve the efficiency of self-healing process of concrete crack.
The authors acknowledge the financial support provided by National Natural Science Foundation of China (No. 51578339).
The authors declare that they have no conflicts of interest associated with this work.
- 1. U.K. Gollapudi, C.L. Knutson, S.S. Bang, and M.R. Islam, Chemosphere. 30[4] (1995) 695-705.
-

- 2. M. Maes, K.V. Tittelboom, and N.D. Belie, Constr Build Mater. 71 (2014) 528-537.
-

- 3. D. Palin, V. Wiktor, and H.M. Jonkers, Cem Concr Res. 73 (2015) 17-24.
-

- 4. J.L. Zhang, R.S. Wu, Y.M. Li, J.Y. Zhong, X. Deng, B. Liu, N.X. Han, and F. Xing, Appl Microbiol Biotechnol. 100[15] (2016) 6661-6670.
-

- 5. F.H. Ramirez-Guadiana, A.J. Meeske, X. Wang, C.D.A. Rodrigues, and D.Z. Rudner, Mol Microbiol. 105[5] (2017) 689-704.
-

- 6. S. Fujinami and M. Fujisawa, Environ Technol. 31[8-9] (2010) 845-856.
-

- 7. S. Faridi and T. Satyanarayana, Environ Sci Pollut Res Int. 25[7] (2018) 6838-6849.
-

- 8. N. Han and F. Xing, J Ceram Process Res. 16[S1] (2015) 14-21.
- 9. J. Spizizen, P NATL ACAD SCI USA. 44[10] (1958) 1072-1078.
-

- 10. J.L. Zhang, J.K. Lu, B. Liu, Q.Y. Liu, F. Jin, M.J. Zhang, Y.R. Liu, Y.J. Song, C.H. Dong, W.Y. Zhang, N.X. Han, X. Deng, and F. Xing, J Ceramic Process Res. 20[S1] (2019) 1-7.
- 11. S. Faridi and T. Satyanarayana, Environ Sci Pollut Res. 23[15](2016) 15236-15249.
-

- 12. Y.S. Lee and W. Park, Appl Microbiol Biotechnol. 102[7] (2018) 3059-3070.
-

- 13. S. Bhaskar, K.M. Anwar Hossain, M. Lachemi, G. Wolfaardt, and M.O. Kroukamp, Cem Concr Comp. 82 (2017) 23-33.
-

- 14. J. Wang, H.M. Jonkers, N. Boon, and N.D. Belie, Appl Microbiol Biotechnol. 101[12] (2017) 5101-5114.
-

- 15. H. Bose and T. Satyanarayana, Front Microbiol. 8 (2017) 1615.
-

- 16. M.G. Sierra Beltran and H.M. Jonkers, J Ceram Process Res. 16[S1] (2015) 33-39.
- 17. D. Palin, H.M. Jonkers, and V. Wiktor, Cem Concr Res. 84 (2016) 1-7.
-

- 18. S.H. Jakhrani, A.Qudoos, A. Rehman, H.G. Kim, I.K Jeon, and J.S.Ryon, J Ceram Process Res. 20[1] (2019) 1-18.
- 19. J. Xu and X. Wang, Constr Build Mater. 167 (2018) 1-14.
- 20. W. Khaliq and M.B. Ehsan, Constr Build Mater. 102 (2016) 63-69.
-

- 21. M. Seifan, A. Ebrahiminezhad, Y. Ghasemi, A.K. Samani, and A. Berenjian, Appl Microbiol Biotechnol. 102[1] (2018) 175-184.
-

- 22. K. Shim, T. Kishi, C. Choi, and T. Ahn, J Ceram Process Res. 16[S1] (2015) 1-13.
- 23. K.V. Tittelboom and N.D. Belie, Materials. 6[6] (2013) 2182-2217.
-

- 24. M. Roig-Flores, F. Pirritano, P. Serna, and L. Ferrara, Constr Build Mater. 114 (2016) 447-457.
-

- 25. B. Dong, G. Fang, W. Ding, Y. Liu, J. Zhang, N. Han, and F. Xing, Constr Build Mater. 106 (2016) 608-617.
-

- 26. G. Ganendra, J. Wang, J. Ramos, H. Derluyn, H. Rahier, V. Cnudde, A. Ho, and N. Boon, Front Microbiol. 6 (2015) 786.
-

- 27. J. Wang, Y.C. Ersan, N. Boon, and N.D. Belie, Appl Microbiol Biotechnol. 100[7] (2016) 2993-3007.
-

- 28. A. Amiri and Z.B. Bundur, Constr Build Mater. 165 (2018) 655-662.
-

- 29. K. Van Tittelboom, J. Wang, M. Araujo, D. Snoeck, E. Gruyaert, B. Debbaut, H. Derluyn, V.Cnudde, E. Tsangouri, D.V. Hemelrijck, and N.D. Belie, Constr Build Mater. 107 (2016) 125-137.
-

- 30. A. Behnood, K. Van Tittelboom, and N.D. Belie, Construct Build Mater. 105 (2016) 176-188.
-

- 31. H. Huang, G. Ye, C. Qian, and E. Schlangen, Mater Design. 92 (2016) 499-511.
-

- 32. J. Feiteira, E. Gruyaert, and N.D. Belie, Constr Build Mater. 102 (2016) 671-678.
-

- 33. D.Y. Zhu, M.Z. Rong, and M.Q. Zhang, Prog Polymer Sci. 49-50 (2015) 175-220.
-

- 34. J. Zhang, B. Mai, T. Cai, J. Luo, W. Wu, B. Liu, N.Han, F. Xing, and X. Deng, Materials (Basel). 10[2] (2017) 116-126.
-

- 35. C. Qian, L. Ren, B. Xue, and T. Cao, Constr Build Mater. 106 (2016) 126-132.
-

 This Article
This Article
-
2019; 20(5): 470-478
Published on Oct 31, 2019
- 10.36410/jcpr.2019.20.5.470
- Received on May 31, 2019
- Revised on Aug 1, 2019
- Accepted on Aug 2, 2019
 Services
Services
- Abstract
introduction
materials and methods
results
discussion
conclusion
- Acknowledgements
- Conflict of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Weizhao Chen , Xu Deng
-
College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518055, PR China
Tel : +86-755-2653-4274
Fax: +86-755-2653-4274 - E-mail: cwz@szu.edu.cn , dengxu@szu.edu.cn






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.