- Microstructure and mechanical properties of yttrium aluminum garnet porous ceramics prepared by different sintering process parameters
Yue Liua, Xueqing Yanga, Kangliang Pengb, Qiong Wangc, Jianzhen Huangc, Zilin Zhanga, Jiang Lua, Hao Xua, Jieguang Songb,c,* and Lin Chena,*
aKey Laboratory for Industrial Ceramics of Jiangxi Province, School of Materials and Chemistry Engineering, Pingxiang University, Pingxiang 337055 China
bResearch Institute of Sponge City, Pingxiang University, Pingxiang 337055 China
cEngineering & Technology Research Center for Environmental Protection Materials and Equipment of Jiangxi Province, School of Materials and Chemistry Engineering, Pingxiang University, Pingxiang 337055 China
Yttrium aluminum garnet (YAG), which possesses excellent properties, is investigated and applied. The sintering technology of YAG porous ceramics is optimized. Results show that the porosity initially increases and then decreases with an increase of increasing temperature rate, the porosity are decreased with an increase of removing carbon temperature, the porosity are decreased with an increase of sintering temperature, the porosity are decreased with an increase of holding time. Meanwhile, the compressive strength constantly exhibits an opposite tendency. The optimization of the sintering technology of YAG porous ceramics is based on porosity and compressive strength. A good sintering technology are an increasing temperature rate of 8 ℃/min, a removing carbon temperature of 800 ℃, removing carbon time of 1 h, a sintering temperature of 1450 ℃ and holding time of 2 h. The porosity of the prepared YAG porous ceramics is 57.4%, and the compressive strength is 8.89 MPa.
Keywords: YAG porous ceramics, sintering technology, porosity, compressive strength
Many types of porous ceramics with high-temperature performance are currently available. The most common porous ceramics are aluminum oxide (Al2O3), silicon carbide (SiC), and silicon nitride (Si3N4). The porous ceramics prepared using Al2O3 have high mechanical strength, low thermal expansion coefficient, good thermal shock resistance, small pressure drop, long service life, fast ignition, and high conversion efficiency in catalysis; however, their performance is unstable under high-temperature conditions (high-temperature bending strength at 1600℃), and they are greatly limited for high-temperature engineering applications [1-3]. Si3N4 porous ceramics have high mechanical strength, high hardness, good high-temperature resistance, and corrosion resistance, which are used extensively in the automotive industry, environmental protection, petroleum industry, and bioindustry. They also have high porosity, low dielectric constant, and dielectric loss, it are suitable for aerospace, military, and chemical industries [4-5].
The porous ceramic materials of a SiC matrix have excellent high-temperature thermal stability, good microporous structure, and strong chemical corrosion resistance. SiC matrix are usually the best choices used as high-temperature filter materials. The application of porous ceramic materials in the field of high-temperature and high-pressure hot gas purification is optimistic [6-8]. However, in the process of preparing porous ceramics from Si3N4 and SiC, single-component materials are difficult to sinter at low temperatures. The needs of industrial production are consequently difficult to meet. Meanwhile, dual-component materials are the indicators of the main crystalline phase. When used under a high-temperature atmosphere, they are easy to oxidize, and some of the properties are missing [9-10]. Therefore, performance advantages are difficult to show when using such engineering materials in a high-temperature atmosphere.
Many types of high-temperature porous ceramics have different degrees of limitations in preparation or application. By contrast, yttrium aluminum garnet (YAG, with a molecular formula of Y3Al5O12) has excellent optical properties, stable chemical properties, and high-temperature performance. YAG is widely used in the preparation and utilization of structural and functional materials [11-13]. YAG materials have good creep resistance, extremely low fracture stress at high temperatures up to 1600℃, and stable performance in a high-temperature oxidation atmosphere. They do not exhibit change in weight for 100 h. Therefore, YAG materials are also one of the candidate materials for use in high-temperature structures [14-15]. Statistical analysis of the literature indicates that YAG is composed of optical functional materials as the main crystal phase. However, studies on porous ceramics are limited.
YAG materials are used to prepare porous ceramic materials and develop unique properties because of their excellent performance in various aspects. Other types of porous ceramic material are improved or replaced in high-temperature catalysis and filtration. This study analyzes YAG porous ceramics. The correlation mechanism between sintering process and their performance and the optimization and adjustment of the sintering process of YAG porous ceramics are investigated. This study provides a reference for preparing high-performance YAG porous ceramics.
The main raw materials used in the experiments were aluminum nitrate Al(NO3)·9H2O (analytical grade), yttrium nitrate Y(NO3)·6H2O (analytical grade), ammonia water, sintering aid, and foaming agent. YAG powder with high purity was prepared by the coprecipitation method. A sintering aid (CaO, 5 wt.%) and a foaming agent (starch, 15 wt.%) were added to the YAG powder for compounding, ball milling, and mixing to obtain composite raw materials. The green body of the YAG porous ceramic material was acquired using dry pressing–forming technology (Φ20 mm×5 mm). Blanks were placed in a ZT-50-22 vacuum carbon tube furnace, sintered to prepare YAG porous ceramic material samples for standby performance test. The density and porosity of the YAG porous ceramics were tested using a LAT5XQK-02 type pore volume densitometer. Their compressive strength was tested using a WDW-E100D microcomputer-controlled electronic universal testing machine. Their microstructure was observed by TESCAN VEGA II scanning electron microscopy.
Effect of heating rate on properties of YAG porous ceramics
Fig. 1 and 2 respectively show the properties and microstructure of the YAG porous ceramics prepared at different heating rates with a sintering temperature of 1400 ℃, carbon removal temperature of 800 ℃, carbon removal time of 1 h, and holding time of 1 h. As the heating rate slows down, the porosity initially increases and then decreases, and the compressive strength shows an increasing trend. The slow heating rate and long sintering cycle leads the particle movement in the green body to cause the volume during the sintering process to diffuse. The surface diffusion and the grain boundary diffusion are sufficient, the more the number and speed of particle migration, the greater the degree of densification of the ceramic. However, if the heating rate is significantly fast, and the high-temperature foaming with short cycle will result in a decrease in porosity [16-17]. As shown in Fig.2, the difference between the two is not evident. The heating rate is fast, the crystal grains are dispersed, the voids are large, and the heating rate is slow. The long high-temperature period produces crystal grains due to the liquid phase. The appearance of solid-phase particles is encapsulated, and the particles form a bonding surface. As the bonding surface is enlarged, a sintered neck is formed. Consequently, the bonding among the crystal grains is strong; the compressive strength is increased, although the difference is small; and the heating rate is preferably 8 ℃/min from the sum of the preparation cycle, porosity, and compressive strength.
Effect of carbon discharge temperature on properties of YAG porous ceramics
Fig. 3 shows the effect of carbon discharge temperature on the properties of YAG porous ceramics at a heating rate of 8 ℃/min, carbon removal time of 1 h, sintering temperature of 1400 ℃, and holding time of 1 h. From the figure, the carbon removal temperature increases, and the porosity decreases; nevertheless, the compressive strength increases. As the temperature increases from room temperature to 1000 ℃ for a long period of time, the carbon is fully formed to create pores. The boundary is diffused and absorbed by it; thus, the pores are reduced, and the sintered body shrinks and densifies, thereby increasing the compressive strength of the sample insignificantly. The energy consumption required to discharge carbon for 1 h at 800 ℃ is less than 1000 ℃. The energy consumption required for carbon removal for 1 h and the mechanical properties of the porous ceramics prepared at 500 ℃ for 1 h are low, which may be due to the incomplete carbon residues in the materials. Fig. 4 shows the schematic of the microstructure of the YAG porous ceramics prepared by different carbon discharge temperatures. The difference between the two is small from the microstructural perspective. The carbon discharge temperature is 800 oC considering porosity, compressive strength, and energy consumption.
Effect of carbon discharge time on properties of YAG porous ceramics
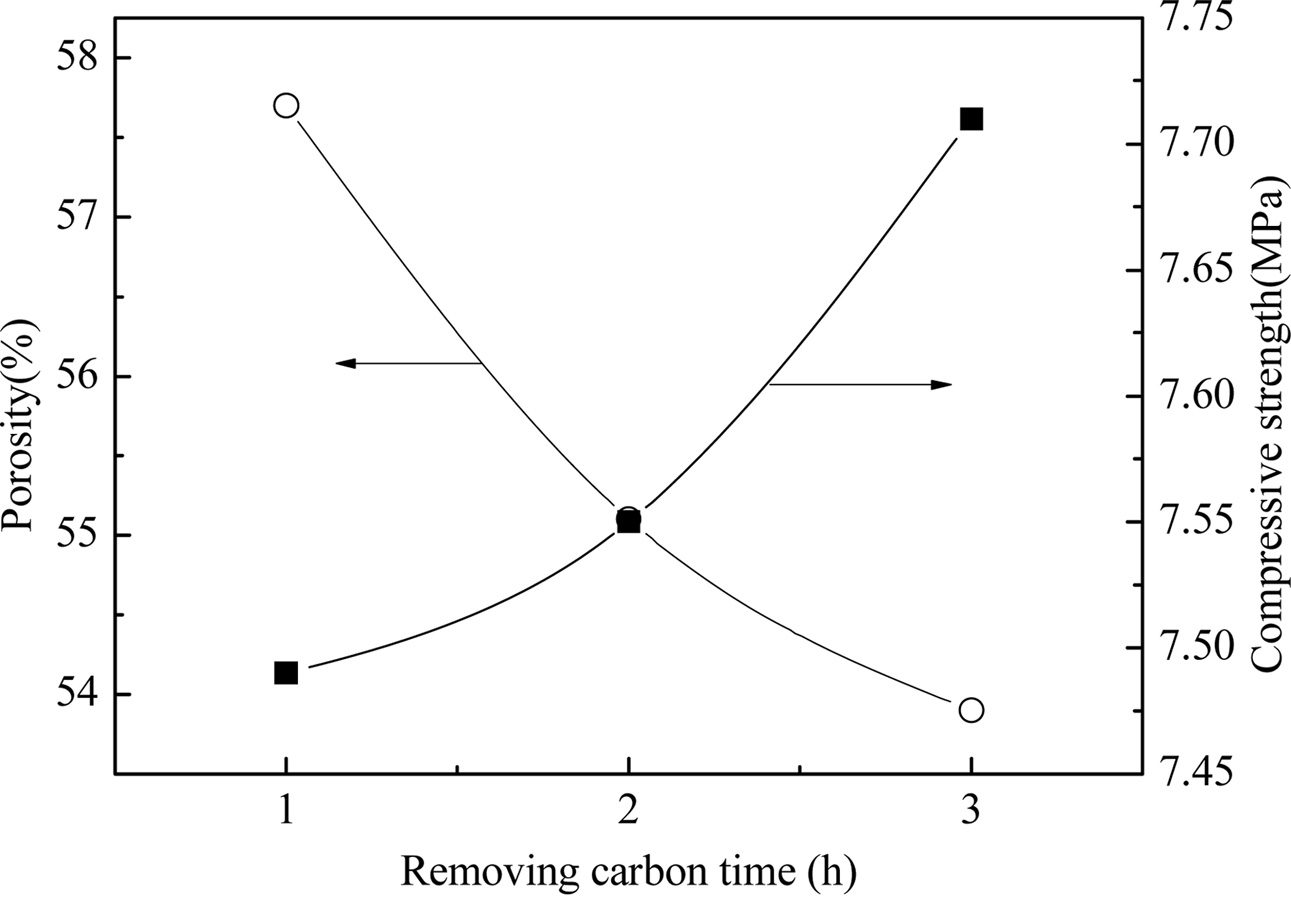
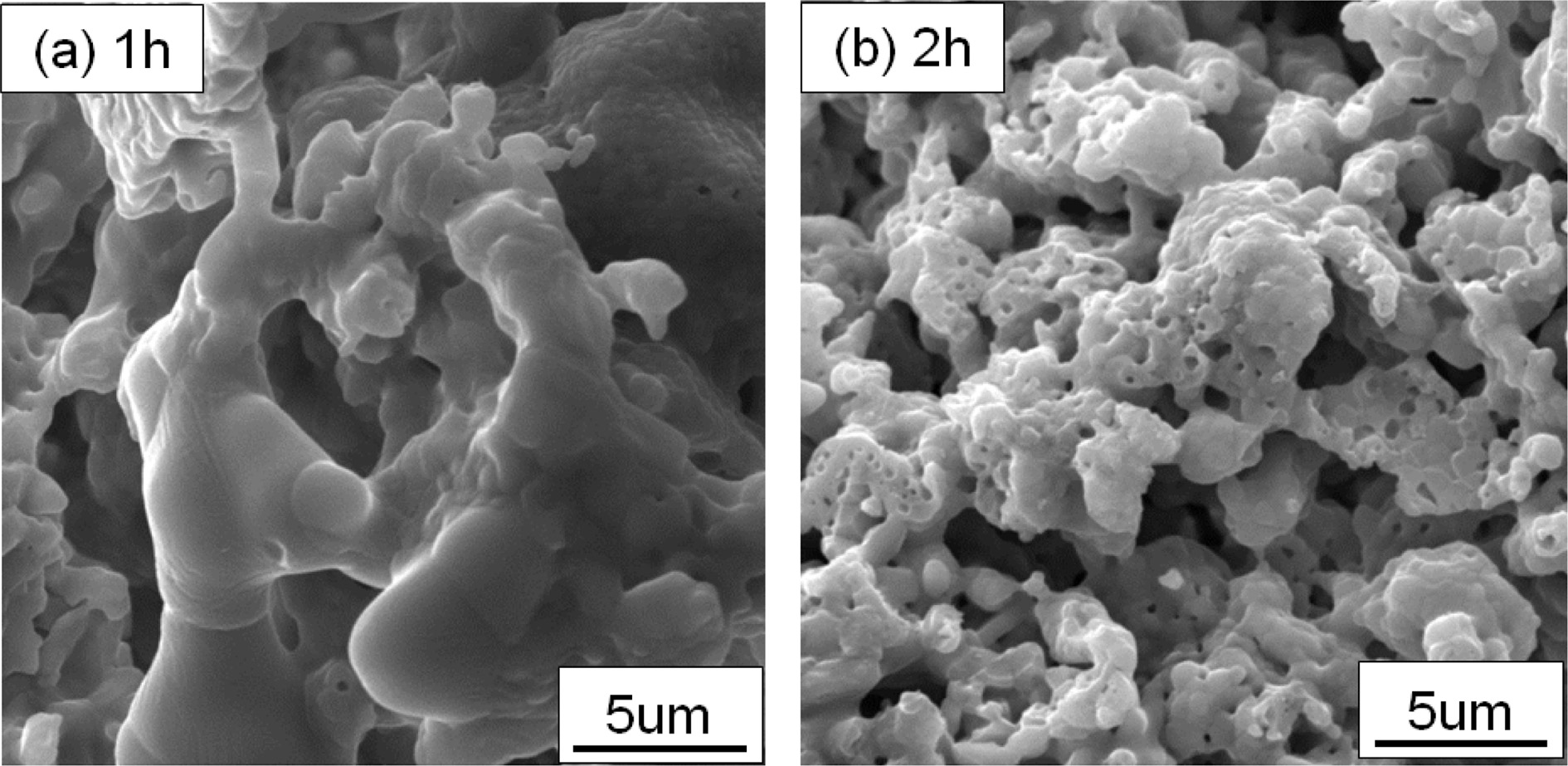
Fig. 5 shows the effect of carbon discharge time on the performance of YAG porous ceramics at a heating rate of 8 ℃/min, carbon removal temperature of 800 ℃, sintering temperature of 1400 ℃, and holding time of 1 h. As the carbon discharge time increases, the porosity shows a downward trend, whereas the compressive strength shows an upward trend. At the same temperature, a longer high-temperature sintering cycle indicates more diffusion and more favorable gas discharge. The molding method is the same, that is, the particle is deposited in the same manner. A longer carbon discharge time implies a more favorable foaming agent in contact with oxygen. The carbon is discharged to form pores. Fig. 6 depicts the microstructure of the YAG sample. Large pores are formed when the carbon is discharged for 1 h. Not only macropores are produced for carbon dioxide for 2 h but also micropores. The large pores appearing for 1 h of carbon discharge are mainly due to the incomplete carbon discharge. As the temperature increases, the liquid phase appears and envelops the gas generated by the high-temperature foaming. The trapped gas forms bubble due to the temperature increase. The thermal expansion eventually forms a large hole. The extension of the carbon removal time reduces the porosity, the improvement of mechanical properties is not evident, and the energy consumption increases. The carbon discharge time must be 1 h for comprehensive performance.
Effect of sintering temperature on properties of YAG porous ceramics
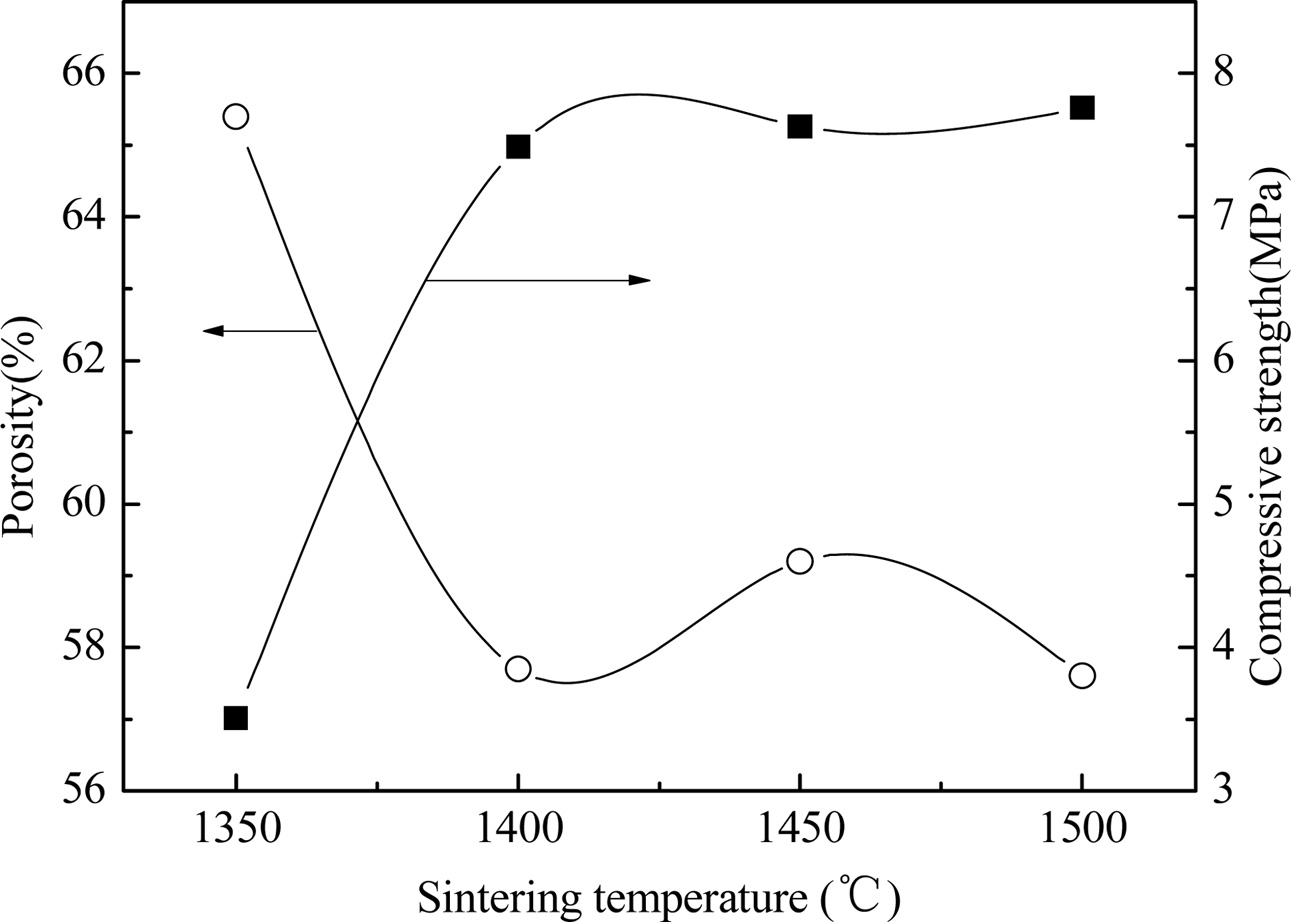
Fig. 7 shows the effect of sintering temperature on the properties of YAG porous ceramics at a heating rate of 8 oC/min, carbon removal temperature of 800 ℃, carbon removal time of 1 h, and holding time of 1 h. Fig. 8 shows the effect of sintering on the sintered ceramic microstructure. The properties of the YAG porous ceramics prepared by sintering the preforms at different temperatures for 1 h are observed. As the sintering temperature increases, the porosity initially increases and then decreases, and the compressive strength increases (Fig. 7). The sintering at a temperature of 1450 oC causes the bubbles to grow; hence, the density decreases, whereas the porosity increases (Fig. 8). The density of the YAG porous ceramic sintered at 1500 oC is higher than that of the YAG porous ceramic sintered at 1450 ℃, and the porosity is also lower. The bubbles are broken to a certain extent because the temperature is considerably high, thereby leaving small bubbles to form small pores and increasing the sintering temperature. The particle diffusion coefficient is increased, which is beneficial to the disappearance of the internal defects of the sintered body or the increase in the sintered neck. The degree of densification of the sample is improved [18-20].
Effect of holding time on properties of YAG porous ceramics
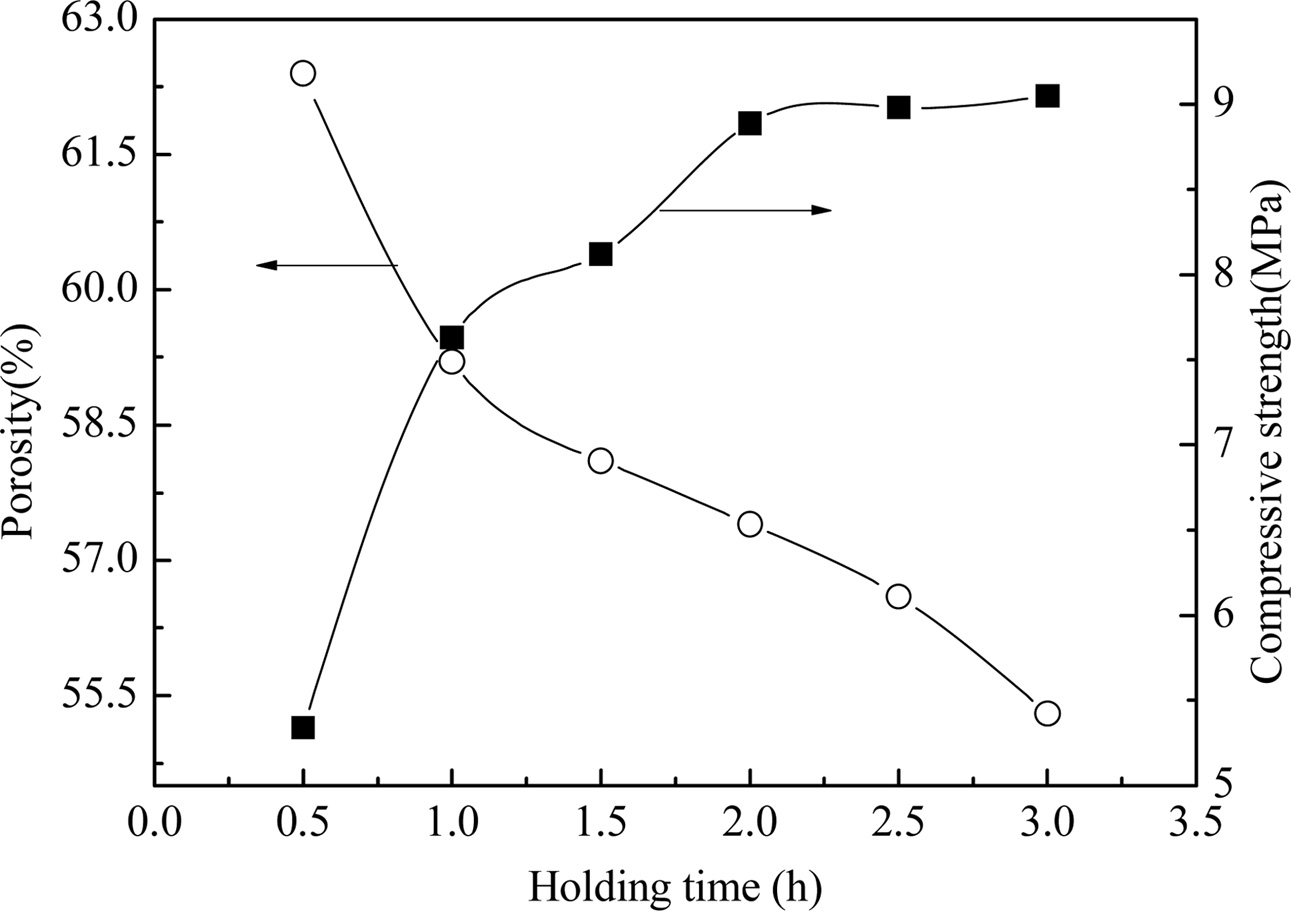
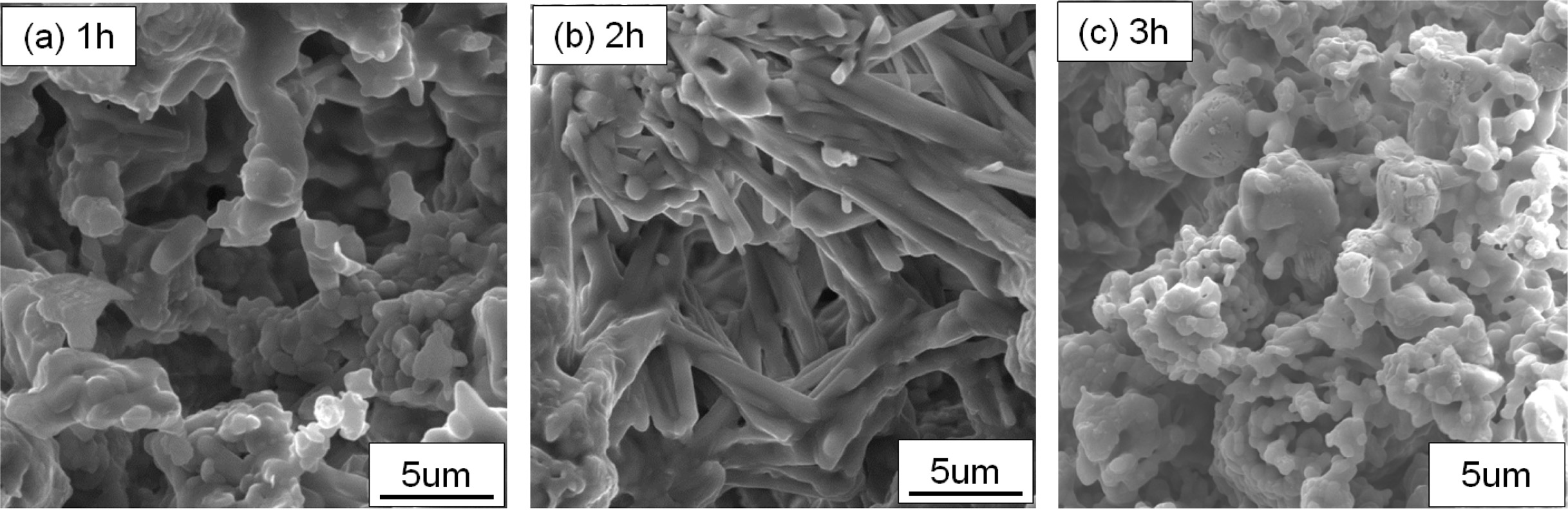
Fig. 9 shows the effect of heating rate on the properties of YAG porous ceramics at a heating rate of 8 ℃/min, carbon removal temperature of 800 oC, carbon removal time of 1 h, and sintering temperature of 1450 oC. Fig. 10 shows the effect of the sample microstructure. As the holding time is prolonged, the porosity is lowered, and the compressive strength is increased. The main reason for this difference is that at the same sintering temperature, a longer sintering time indicates a longer sintering cycle, more diffusion among the particles, and more time for the grains to grow. The grain remains granular when the heat is kept for 1 h, and no regular pores are formed (Fig. 10). When the holding time is 2 h, the crystal grains are rod shaped because of the long sintering time and granular grains. The rod-like grains are interlaced with one another, which is advantageous for improving the mechanical strength of the sample. Under the condition of ensuring porosity, the compressive strength increases considerably. Accordingly, the holding time is preferably 2 h.
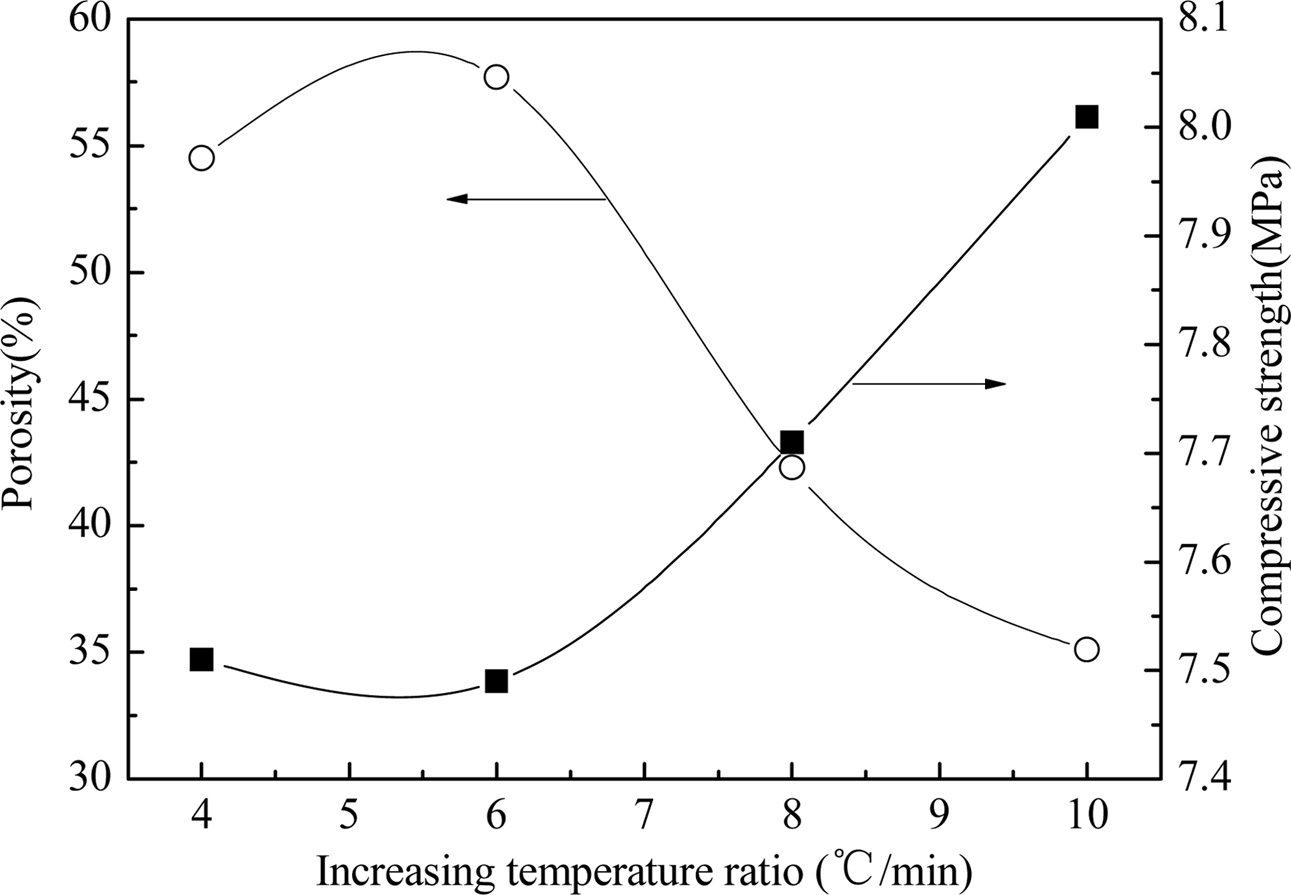
|
Fig. 1 Fig. 1. Properties of prepared YAG porous ceramics under different increasing temperature ratio. |
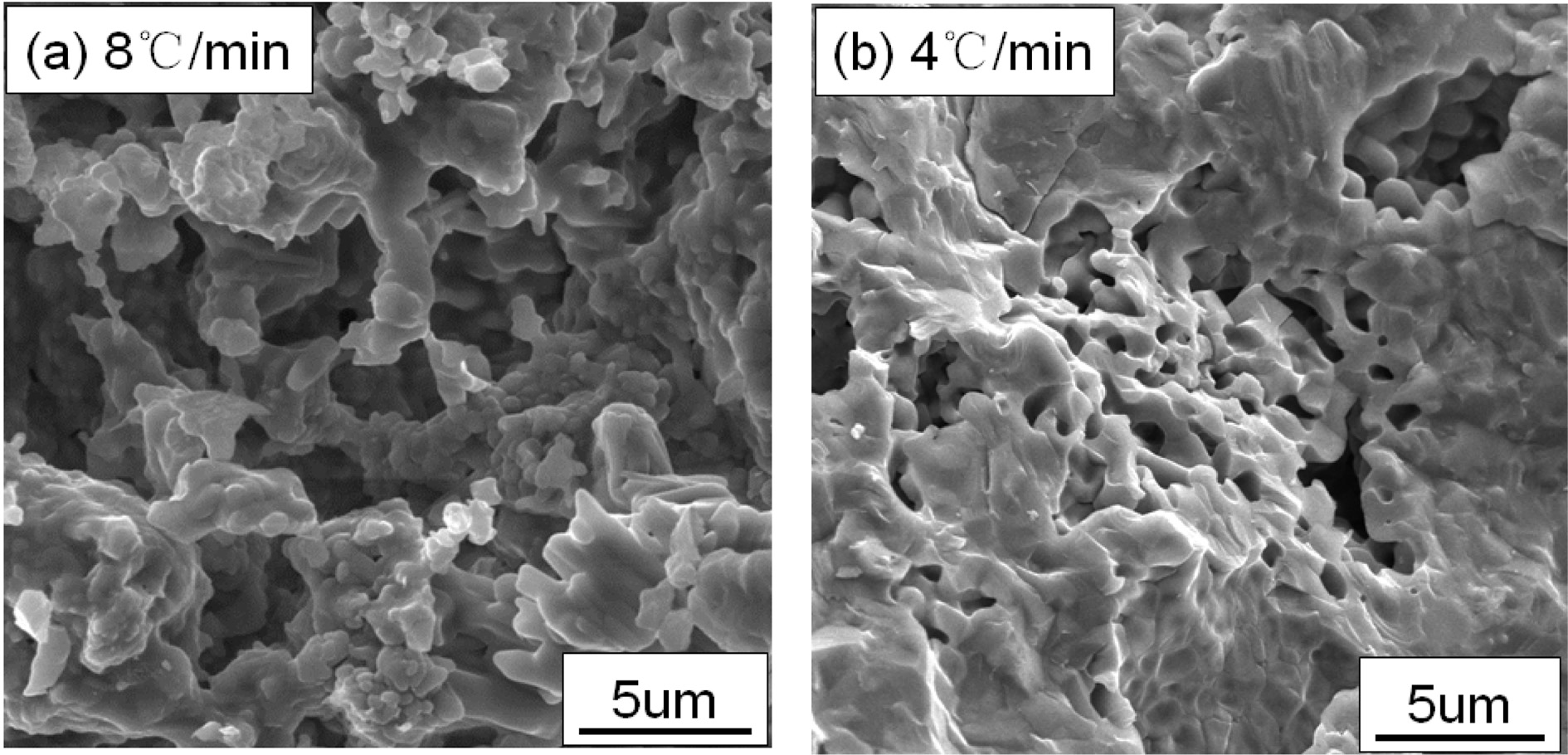
|
Fig. 2 Fig. 2. Microstructure of prepared YAG porous ceramics under different increasing temperature ratio. |
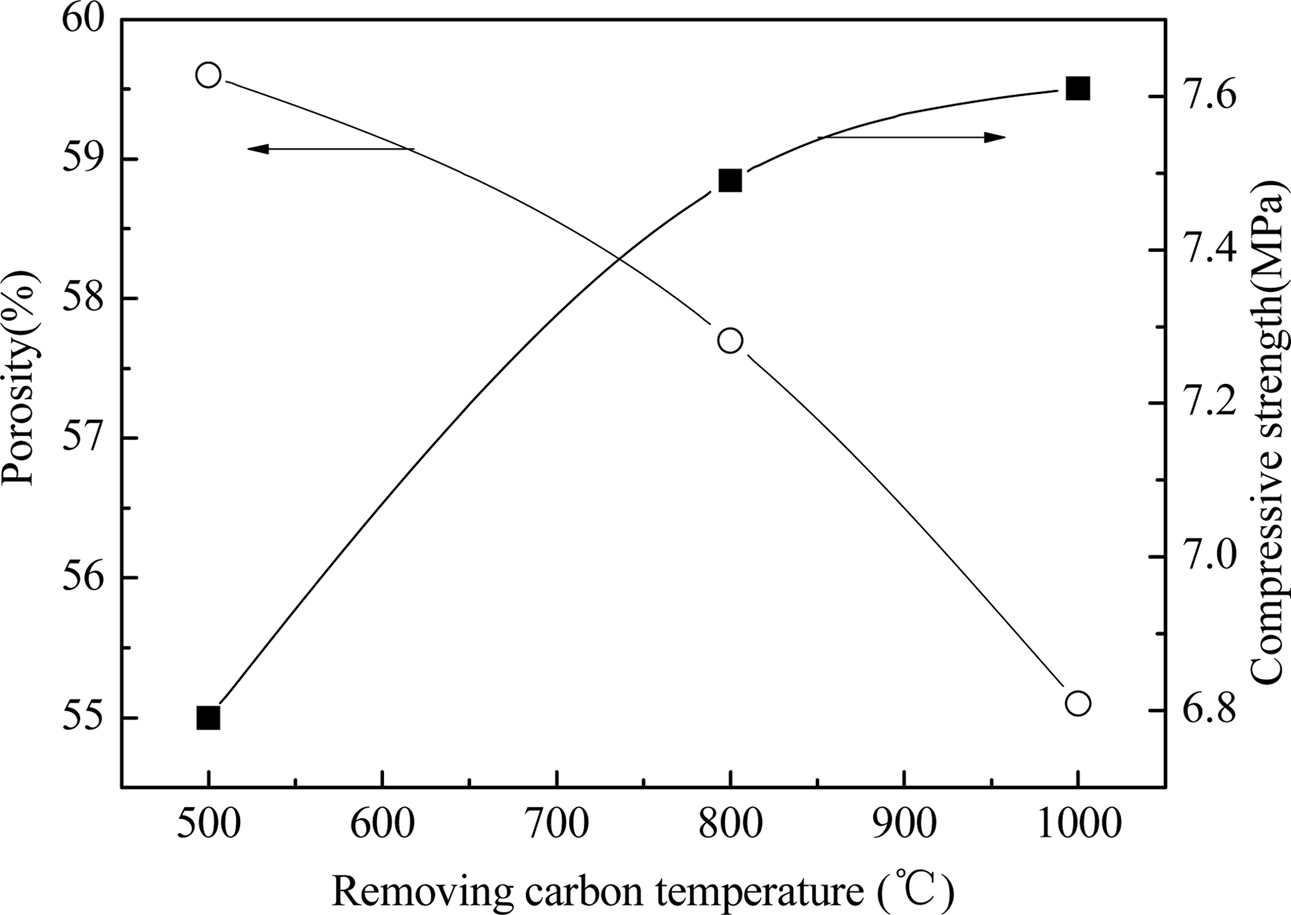
|
Fig. 3 Fig. 3. Properties of prepared YAG porous ceramics under different removing carbon temperature. |
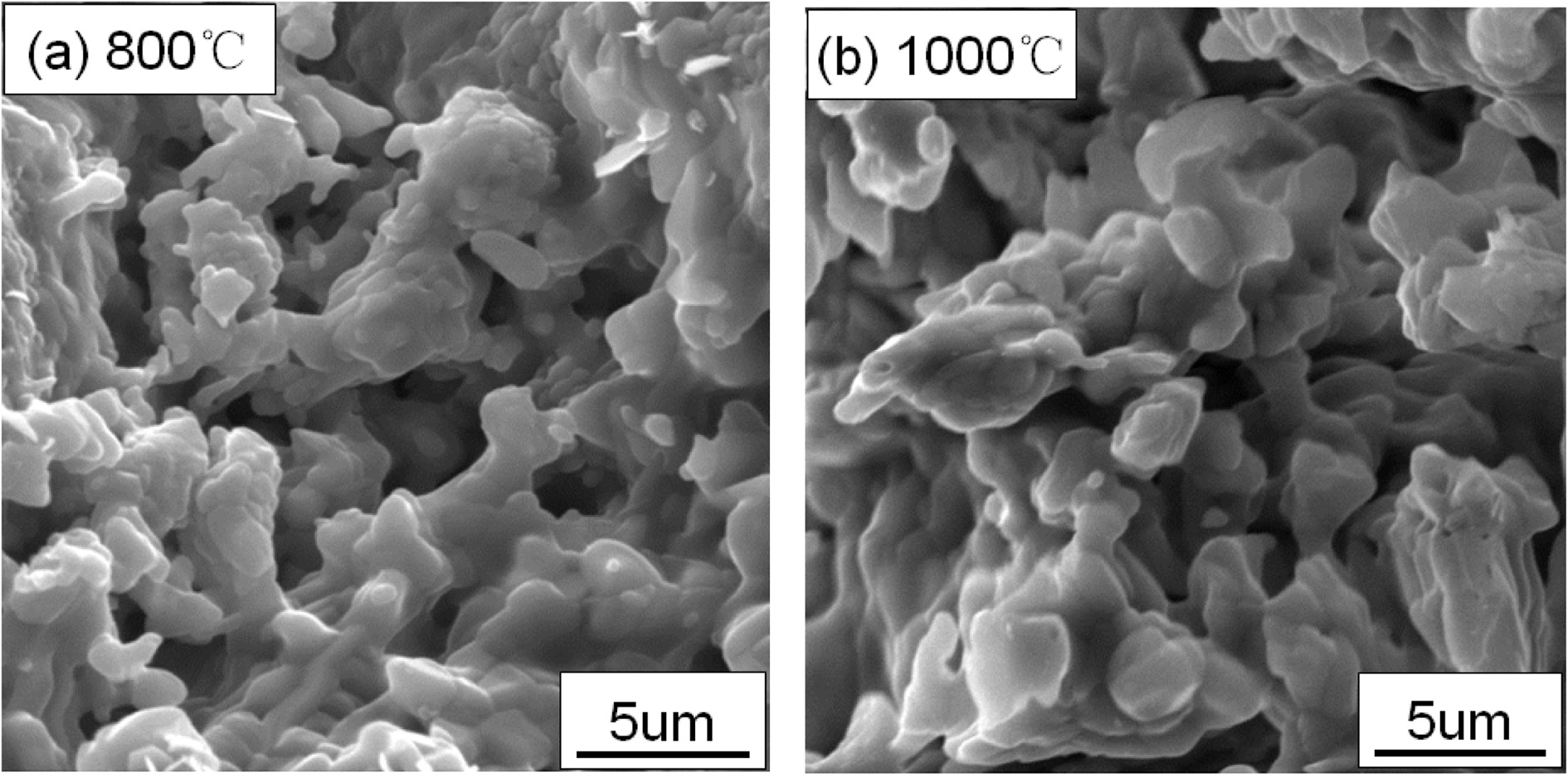
|
Fig. 4 Fig. 4. Microstructure of prepared YAG porous ceramics under different removing carbon temperature. |
|
Fig. 5 Fig. 5. Properties of prepared YAG porous ceramics under different removing carbon time. |
|
Fig. 6 Fig. 6. Microstructure of prepared YAG porous ceramics under different removing carbon time. |
|
Fig. 7 Fig. 7. Properties of prepared YAG porous ceramics under different sintering temperature. |

|
Fig. 8 Fig. 8. Microstructure of prepared YAG porous ceramics under different sintering temperature. |
|
Fig. 9 Fig. 9. Properties of prepared YAG porous ceramics under different holding time. |
|
Fig. 10 Fig. 10. Microstructure of prepared YAG porous ceramics under different holding time. |
Through data results and analysis, the conclusions can be obtained as follows, the porosity initially increases and then decreases with an increase of increasing temperature rate, the porosity are decreased with an increase of removing carbon temperature, the porosity are decreased with an increase of sintering temperature, the porosity are decreased with an increase of holding time. Meanwhile, the compressive strength constantly exhibits an opposite tendency. The optimization of the sintering technology of YAG porous ceramics is based on porosity and compressive strength. A good sintering technology are an increasing temperature rate of 8℃/min, a removing carbon temperature of 800℃, removing carbon time of 1 h, a sintering temperature of 1450℃ and holding time of 2 h. The porosity of the prepared YAG porous ceramics is 57.4%, and the compressive strength is 8.89 MPa.
The authors are thankful for the financial support provide by the Science and Technology Found of the Educational Department of Jiangxi Province, China (GJJ181107), the Platform Construction Found of Science and Technology Department of Jiangxi Province (20161BCD40011) and the Research Project of Teaching Reform for Higher Education of Jiangxi Province (JXJG-18-22-2).
- 1. Z. Feng, X. Lv, T.H. Wang, J. Porous Mater. 25 (2018) 189-198.
-

- 2. H. Wang, X. Zhang, N. Wang, Y. Li, X. Feng, Y. Huang, Sci. Adv. 3 (2017) 1-9.
-

- 3. N. Arai, K. T. Faber. Script. Mater. 162 (2019) 72-76.
-

- 4. B. Zhang, H.M. Huang, X.L. Lu, J. Eur. Ceram. Soc. 39 (2019) 1775-1780.
-

- 5. B. Ren, Y.L. Wang, J.J. Liu, X.Y. Zhang, J.L. Yang, Ceram. Int. 45 (2019) 6581-6584.
-

- 6. P.Q. Zhao, Q.G Li, R.J Yi, Z. Wang, S.M. Dong. J. Alloy. Compd. 748 (2018) 36-43.
-

- 7. T.D. Afolabi, M. Ariff, A.H. Mazlan, Int. J. Appl. Ceram. Tech. 15 (2018) 1060-1071.
-

- 8. S. Stub, E. Vollestad, T. Norby, J. Mater. Chem. A 6 (2018)8265-8270.
-

- 9. S. Baitalik, S.K. Dalui, N. Kayal, J. Mater. Sci. 53 (2018) 6350-6365.
-

- 10. J.G. Song, L. Chen, C.L. Pang, J. Ceram. Process. Res. 19 (2018) 11-14.
- 11. J. Kraxner, J. Chovanec, K. Haladejova, I. Petrikova, D. Galusek, Mater. Letts. 204 (2017) 181-183.
-

- 12. G.H. Zhou, G.C. Xu, J. Liu, Y.K. Tian, X.P. Gu, Int. J. Adv. Manuf. Tech. 95 (2018) 1677-1684.
-

- 13. J.G. Song, M.H. Xu, F. Wang, J. Ceram. Process. Res. 14 (2013) 376-379.
-

- 14. J. Hostasa, V. Necina, T. Uhlírova, V. Biasini, J. Eur. Ceram. Soc. 39 (2019) 53-58.
-

- 15. X. Wang, Y.J. Zhong, D. Wang, L.C. Sun, B.L. Jiang, J.Y. Wang, J. Am. Ceram. Soc. 100 (2017) 1-7.
-

- 16. N. Adilah A. Hassan, S. Ahmad, R.S. Chen, F.D. Zailan, D. Shahdan, Mater. Today 7 (2019) 601-606.
-

- 17. C. Settgast, Y.R. Klemm, J. Hubalkova, M. Abendroth, H. Biermann, J. Eur. Ceram. Soc. 39 (2019) 610-617.
-

- 18. N. Chawake, P. Ghosh, L. Raman, A.K. Srivastav, R.S. Kottada, Script. Mater. 161 (2019) 36-39.
-

- 19. H. Shao, A.P. Wu, Y.D. Bao, Y. Zhao, G.S. Zou, L. Liu, Mater. Sci. Eng. A 724 (2018) 231-238.
-

- 20. Z.J. Yu, X. Lv, S.Y. Lai, L. Yang, W.J. Lei, X.G. Luan, R. Riedel, J. Adv. Ceram. 8 (2019) 112-120.
-

 This Article
This Article
-
2019; 20(4): 436-441
Published on Aug 31, 2019
- Received on Apr 30, 2019
- Revised on Jun 25, 2019
- Accepted on Jul 10, 2019
 Services
Services
- Abstract
introduction
experimental materials and methods
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jieguang Song b,c and Lin Chen a
-
aKey Laboratory for Industrial Ceramics of Jiangxi Province, School of Materials and Chemistry Engineering, Pingxiang University, Pingxiang 337055 China
bResearch Institute of Sponge City, Pingxiang University, Pingxiang 337055 China
cEngineering & Technology Research Center for Environmental Protection Materials and Equipment of Jiangxi Province, School of Materials and Chemistry Engineering, Pingxiang University, Pingxiang 337055 China
Tel : +86-799-6682178
Fax: +86-799-6682171 - E-mail: sjg825@163.com(J. G. Song) rymw27@163.com (L. Chen






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.