- Manufacturing properties of γ-dicalcium silicate with synthetic method
Zheng-xin Chena, Han-seung Leeb and Hyeong-Kyu Choc,*
aDepartment of architectural system engineering, Hanyang university, Ansan 15588, Korea
bDepartment of architectural engineering, Hanyang university, Ansan 15588, Korea
cEnergy and Environmental Division, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Korea
γ-dicalcium silicate(γ-C2S) is known as a polymorphism of belite. Due to its high CO2 fixed capacity and the low CO2 emission production process, γ-C2S has attracted more and more attention of researchers. For the further development of application ofγ-C2S in building construction industry. In this study, we aim to investigate the method for synthesizing high purity of γ-C2S. The influence of different raw materials and calcination temperatures on the purity of γ-C2S was also evaluated. Several Ca bearing materials were selected as the calcium source, the materials which’ s main component is SiO2 were used as the silicon source. Raw materials were mixed and were calcined under different temperatures. The results reveal that the highest purity could be obtained using Ca(OH)2 and SiO2 powder as raw materials. And for the practical application, a relatively economic synthesis method using natural mineral materials- limestone and silica sand as raw materials was developed, by this method, the purity of the synthetic γ-C2S was 77.6%.
Keywords: Steel; γ-dicalcium silicate, Carbonation, CO2 capture
Although olivine group mineral γ-C2S has a poor hydration performance, it has a strong CO2 fixation performance, which has attracted the attention of many researchers in recent years [1-4]. In the presence of water, γ-C2S can react quickly with CO2 to produce CaCO3 and SiO2 gel [5-8]. In recent years, researchers have developed fast, hard and high-strength materials by utilizing the rapid reaction of γ-C2S with CO2 and the cementitious ability of carbonation products, taking γ-C2S as the main component by using the carbonization curing method. Besides, Higuchi et al. [9] added fly ash and γ-C2S as main materials to concrete and developed a new type of concrete called CO2-SUICOM. This new type of concrete is maintained in the exhaust treatment room full of exhaust gas at power plant, with a concentration of 15%-20%. After 28 days of curing, the strength of CO2-SUICOM reached 21 N/mm2, which was close to or even higher than OPC concrete. Guan et al. [4] studied that the mortar test block made with γ-C2S could reach 60 MPa of strength within 5 hours after high-concentration of CO2 curing. Meanwhile, the amount of CO2 released by γ-C2S in the manufacturing process is lower than OPC. The application of γ-C2S was convinced to have a relatively good prospect as it could reduce low CO2 emission compared to OPC.
C2S has five crystal form, which are α, αH’, αL’, β and γ. For pure C2S, crystal form of α-C2S, αH’-C2S, αL’-C2S and β-C2S can’s exit steadily in constant temperature,with the trend of transforming towards γ-C2S[10]. Therefore, γ-C2S can be produced according to the polymorphs conversion mechanism between various crystal forms of C2S. The polymorphs of C2S and conversion relationship were shown in Fig. 1. Appropriate Ca ion supply material and Si supply material powder are selected and mixed in a certain proportion. Then, the mix was heated to a desired temperature to form a relatively unstable α-C2S. Next, α-C2S was slowly converted to γ-C2S as it was cooled at a room temperature. Theoretically, the density of β-C2S is 3.05 whereas γ-C2S is 2.97 [11-12]. Therefore, in the process of transformation from β-C2S to γ-C2S, the volume of cement clinker ball will expand and then pulverize, which is known as “dusting phenomenon” [14-15]. Thus, the grinding energy consumption in the manufacturing process can be reduced [1].
Kriskova et al. [15] used analytical grade CaCO3 and SiO2 powder to calcine at 1450 ℃ for 20 hours, with a cooling speed of 1 ℃/ min. The content of γ-C2S and β-C2S of synthetic sample is 88 ± 4% and 12 ± 4%, respectively. Liu et al. [16] mixed analytical grade CaCO3 and SiO2 powder and calcined at 1350 ℃. In the synthetic samples, γ-C2S is the dominant crystal form. Mu et al. [17] used pure Ca(OH)2 and SiO2 to be calcined at 1100 ℃, 1200 ℃, 1300 ℃ and 1400 ℃ in a mole ratio of 2:1. Results show that γ-C2S becomes the primary component under 1300 ℃, and γ-C2S’s purity can be maximized when samples were calcined under 1400 ℃. It can be seen that pure chemical reagents are recommended to use with a minimum temperature of 1300 ℃ to achieve a higher purity.
Higuchi et al. [9] mixed SiO2 powder with an industrial by-product powder which is Ca(OH)2. After calcined at 1450 ℃, the content of γ-C2S and β-C2S is 93.7% and 1.4%, respectively. The CO2 emission of γ-C2S synthesized was about 1/5 of the OPC. Guan et al. [4] used limestone and shale powder as raw materials, added a certain amount of water and molded under pressure. The samples were calcined under 1300 ℃ with a 95% of C2S. Japan which has carried out relevant researches earlier has entered into the stage of mass production. Previous researchers [18] take industrial materials by-products and calcined in rotary kiln for mass production.
In this study, the influence of different materials and calcining temperatures on the purity of γ-C2S was investigated. For the practical purposes of γ-C2S, a relatively economic synthesis method using nature mineral materials is conducted.
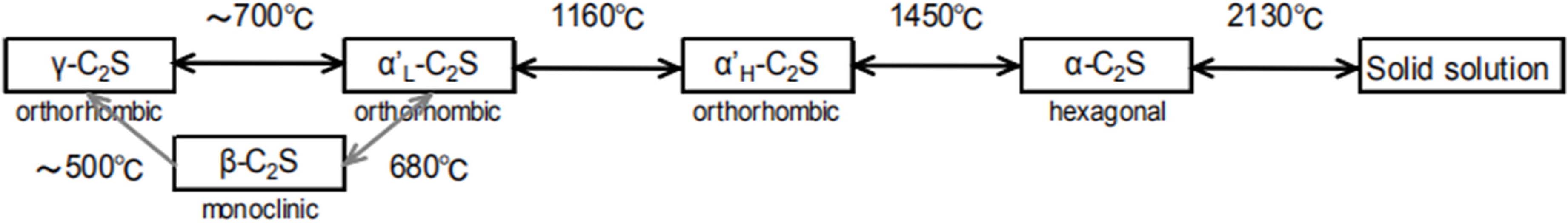
|
Fig. 1 Polymorphs of Dicalcium Silicate. |
In order to investigate the effect of different raw materials on purity of γ-C2S, analytical grade CaO powder, Ca(OH)2 powder and CaCO3 powder were selected to provide Ca ions. Analytical grade SiO2 powder was used as the source of Si ions. Next, both of the ions were mixed properly with Ca/Si ratio of 2:1. The schematic diagram of calcination process was shown in Fig. 2. The rate of temperature was set at 10 oC/min, and then temperature was maintained at 1450 ℃. After calcination, samples were cooled at room temperature. Then, the S1 sample synthesized by using CaO powder and SiO2 powder, S2 synthesized by using Ca(OH)2 powder and SiO2 powder, and S3 synthesized by using CaCO3 powder and SiO2 powder, samples of γ-C2S are obtained respectively. The analytical grade chemical reagent are manufactured by Deajung chemicals & materials co., ltd, Korea. The purity of CaO powder, Ca(OH)2 powder and CaCO3 powder is above 97%, 95% and 98% respectively. SiO2 powder is extra pure. The composition of synthetic γ-C2S samples was detected by Q-XRD.
CaCO3 has a relatively lower value than Ca(OH)2. In order to improve the economic efficiency of γ-C2S in practical applications, it attempted to improve the purity of synthetic γ-C2S in laboratory of Ssangyong cement industrial co., ltd. Analytical grade of CaCO3 and SiO2 powder were used as raw materials. The Ca/Si ratio was selected at 1.86. A certain amount of water is added to the mixed powder to transform it into a sphere. Then, the samples were calcined in a furnace. After the samples were heated at 900 ℃ with a temperature increase rate of 10 ℃/min for 1 hour, the samples were continuously heated till up to 1450 ℃, and temperature was maintained at 1450 ℃ for 1 hour. After cooling process, the synthetic sample- S4 was obtained. The schematic diagram of synthesis process of S4 is shown in Fig. 3. The composition of S4 was detected by Q-XRD and XRF.
As synthesis of γ-C2S using pure chemical reagents is expensive and not conducive to mass production and practical application. Natural minerals was used as raw materials. The high purity limestone powder and silica sand powder were used and mixed with Ca/Si ratio of 1.86. Chemical composition of limestone powder and silica sand powder were shown in Table. 1 and Table. 2, respectively.
After it was added with 10% of water, the mix was pressed into the cylinder mold and dried for 3 h at 100 ℃. Then, 3 groups of cylindrical samples were put in a furnace at 900 ℃ with a rate of 10 ℃/min for 1 hour. Then, the samples were continuously heating at 1200 ℃, 1300 ℃, and 1400 ℃ of temperature for 1.5 hour, and after cooling process, S5, S6, and S7 were obtained, respectively. The schematic diagram of synthesis process of synthetic samples using nature materials is shown in Fig. 4. The composition of S5, S6, and S7 was detected by Q-XRD.
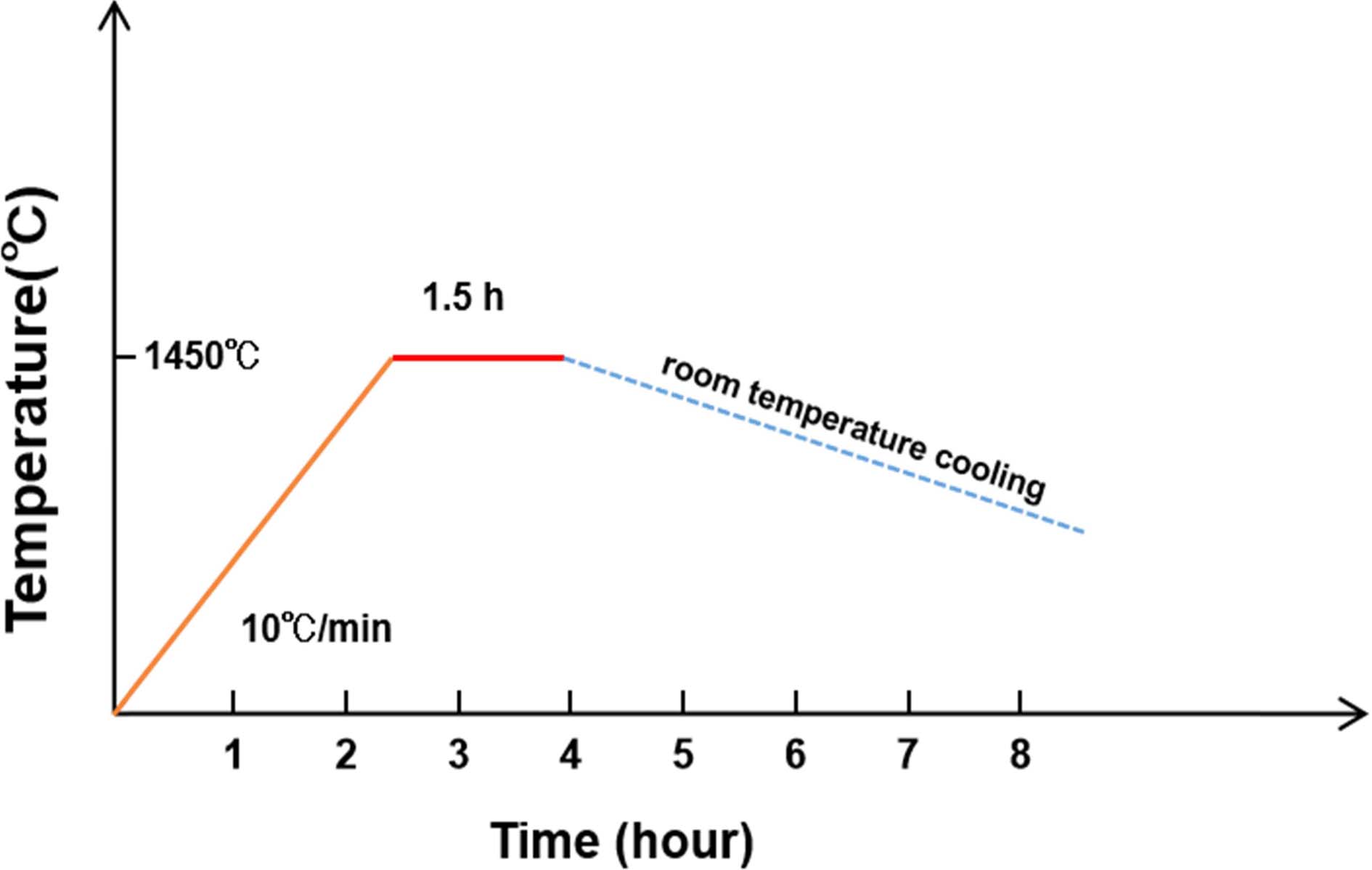
|
Fig. 2 The schematic diagram of calcination process of S1, S2, and S3. |

|
Fig. 3 Synthesis process of S4. |
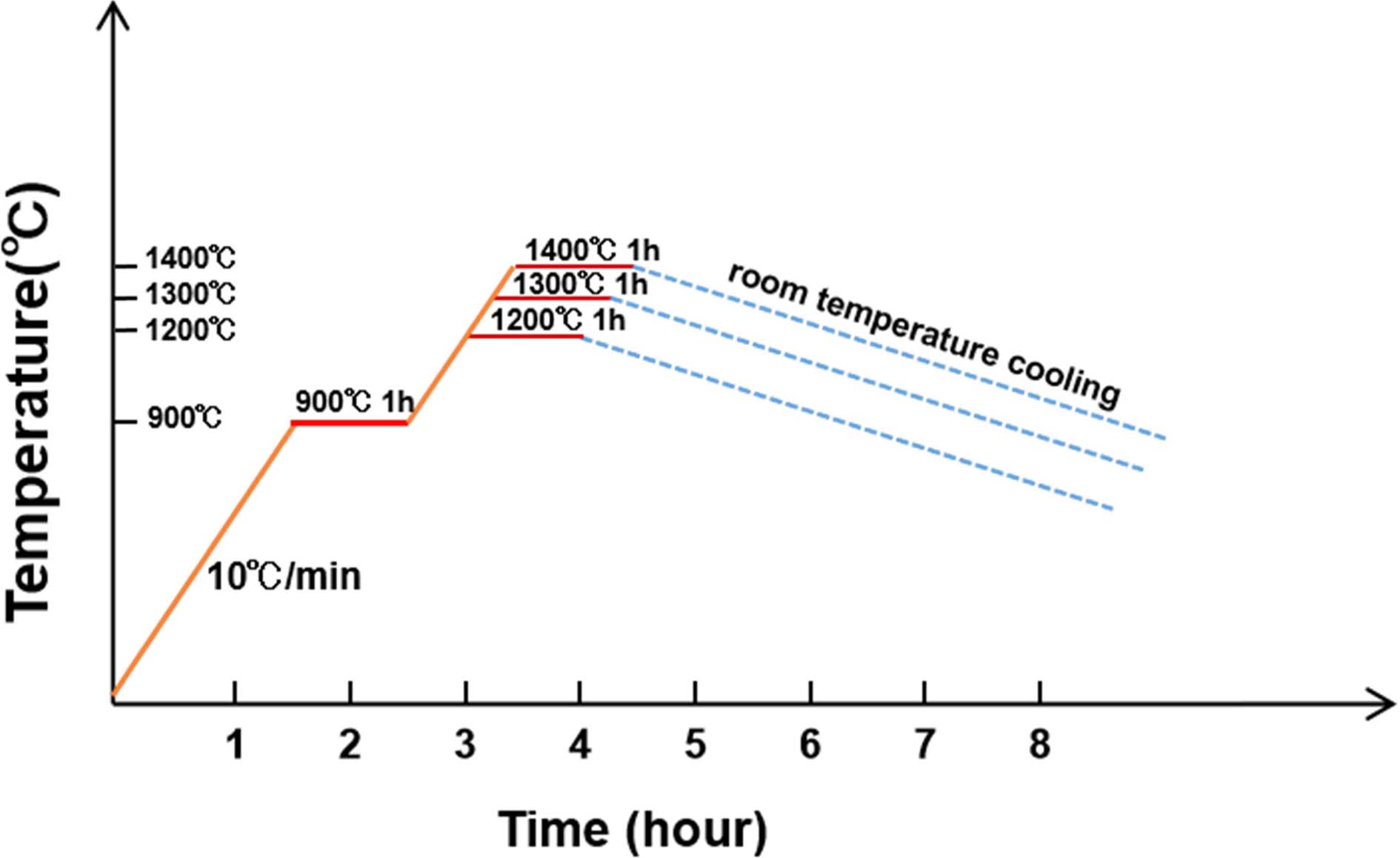
|
Fig. 4 The schematic diagram of calcination process of S5, S6, and S7. |
|
Table 3 Purity of synthesis γ-C2S samples by using analytical grade materials (wei. %). |
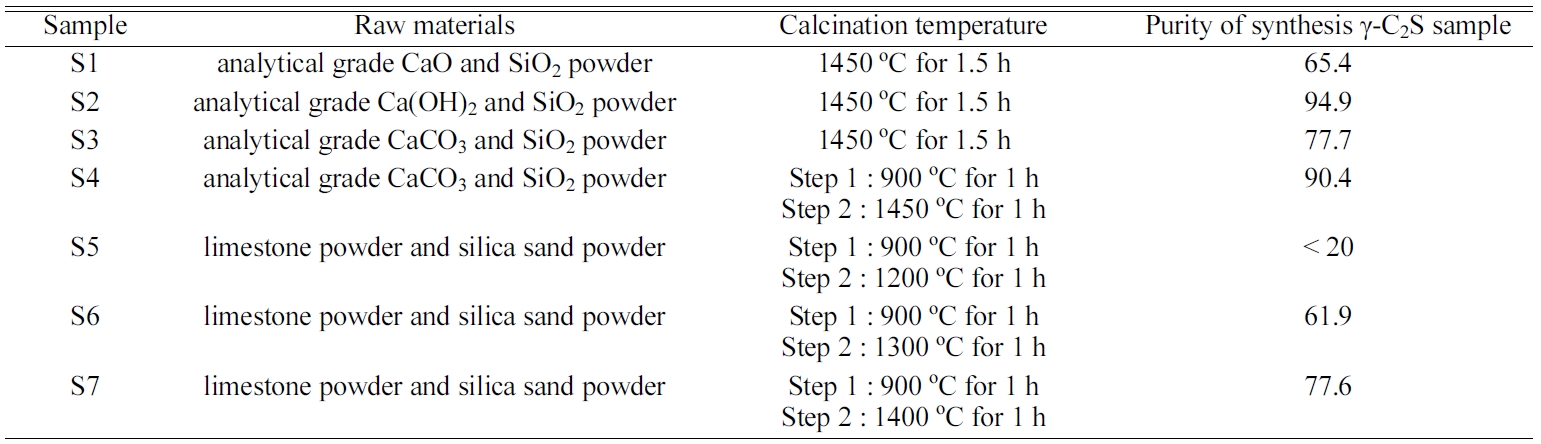
The purity of synthesis γ-C2S samples are shown in Table. 3. For the S1, S2, S3, result shows that under the same experimental conditions, maximum purity of γ-C2S samples synthesized by using Ca(OH)2 powder is 94.9%. Besides, samples synthesized by using CaCO3 powder shows a relatively high purity which is 77.7%. Synthetic samples using CaO powder has the lowest purity, which is caused by the low activity of CaO.
The purity of synthetic γ-C2S sample- S4 by using CaCO3 powder is 90.4%. Comparing with the S3, although the calcination temperature was deceased, the purity was improved. This was due to the Ca/SiO ratio which is 1.86 that controlled the formation of free CaO. In additional, the presence of water reduced the distance between the powder particles and increase the contact area. Furthermore, calcining at 900 ℃ for 1 hour made the CaCO3 powder to be fully decarbonized then the synthesis reaction was enhanced.
For the synthetic samples using limestone and silica sand powders -S5, S6 and S7, with the increase of temperature, the formation of high purity γ-C2S was improved which can enhanced the reaction degree. The purity of γ-C2S synthesized at 1400 ℃ is 77.6% which is the highest. Besides, γ-C2S synthesized at 1200 ℃ contains a large number of impurities such as unreacted CaO with a main product of β-C2S. The purity of samples synthesized at 1300 ℃ temperature is 69.1%. From the perspective of economy, it is more suitable for practical application.
In this study, the influence of different materials and synthetic methods on the purity of γ-C2Swas explored. The main conclusions are as follow:
Under laboratory conditions, synthesize γ-C2S by using Ca(OH)2 as calcium resource can obtain synthetic γ-C2S with the highest purity.
Selecting Ca/Si ratio at 1.86, addition of water and fully decarbonization can enhance the purity of synthetic γ-C2S by using CaCO3 powders.
Using natural mineral materials and heat at relatively high temperature can obtain γ-C2S with relativity high purity. This synthesis method using the economical materials as raw materials which provided an idea for mass amount manufacturing of γ-C2S, and prepared the conditions for future practical applications.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education(2017R1D1A1B03036087).
- 1. Mu. Y, Xue. G, Zhao. S, Huang. X, Wang. F, J. Chinese Ceramic Soc. 45 (2017) 1198-1203.
- 2. T. Saito, S. Khamhou, T. Yumoto, and N. Otsuki, J. Adv. Con. Tech. 9[3] (2011) 223-230.
-

- 3. T. Saito, E. Sakai, M. Morioka and M. Daimon, J. Adv. Con. Tech. 5[3] (2007) 333–341.
-

- 4. X. Guan, S. Liu, C. Feng, M. Qiu, Const. and Bui. Mater. 114 (2016) 204-207.
-

- 5. J. Chang, Y. Fang and X. Shang, Mater. and Struc. 49 (2016) 4417-4424.
-

- 6. K. Watanabe, K. Yokozeki, R. Ashizawa, N. Sakata, M. Morioka and E. Sakai, Waste Manage 26 (2006) 752-757.
-

- 7. S. Tsuyoshi, S. Etsuo, M. Minoru and O. Nobuaki, J. Adv. Con. Tech. 8 (2010) 273-280.
- 8. S. Khamhou, S. Tsuyoshi, O. Nobuaki and Y. Tatsuya, J. Soc. Mater. Sci. 61 (2012) 299-307.
- 9. T. Higuchi, T. Morioka, I. Yoshioka and K. Yokozeki, Const. and Build. Mater. 67 (2014) 338-343.
-

- 10. R. Sakurada, A. Singh, B. Yakobson, M. Uzawa, and Y. Kawazoe, in Proceedings of the 33rd Conference on Our World In Concrete & Structures, August 2008, edited by K.C.G Ong, C.T. Tam, T.H. Tan (Singapore CI-Premier Pte. Ltd, 2008) p.25.
- 11. S. Lee, K. Kim and M. Song, J. Korea Cer. Soc. 53[2] (2016) 194-199.
-

- 12. M. Lesti, C. Tiemeyer and J. Plank, Cem. And Con. Res. 45 (2013) 45-54.
-

- 13. K. Watanabe, K. Yokozeki, R. Ashizawa, N. Sakata, M. Morioka, E. Sakai, M. Daimon, Waste Manage 26 (2006) 752-757.
-

- 14. M. Sung, H. Cho and H. Lee, J. Korea Inst. Build. Const. 15[3] (2015) 281-289.
-

- 15. L. Kriskova , Y. Pontikes , F. Zhang, Ö. Cizer, P. Jones, K. Balen, and B. Blanpain. Cem. and Con. Res. 55 (2014) 59–68.
-

- 16. L. Songhui, G. Xuemao, Q. Man and F. Chunhua, J. Chinese Ceramic Soc. 45 (2016) 658-662.
- 17. Y. Mu, G. Xue, S. Zhao, X. Huang and F. Wang, J. Chinese Ceramic Soc. 45 (2017) 1197-1203.
- 18. M. Morioka, K. Yamamoto, T. Torichigal and K. Yokozeki, Cem. Sci. and Con. Tech. 64 (2011) 29-34.
-

 This Article
This Article
-
2019; 20(S1): 109-112
 Services
Services
- Abstract
introduction
synthetic methods ofγ-c2s
γ-c2ssynthesis experiment
result and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Hyeong-Kyu Cho
-
Energy and Environmental Division, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Korea
Tel : +82-55-792-2472
Fax: 82-55-792-2469 - E-mail: hkcho@kicet.re.kr








 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.