- Sodium titanate as an infrared reflective material for cool roof application
Mahboob Ullah, Hee Jung Kim, Jae Gu Heo, Dong Kyu Roh and Dae-Sung Kim*
Energy Environment Division, Korea Institute of Ceramic Engineering and Technology (KICET), 101, Soho-ro, Jinju-si, Gyeongsangnam-do 52851, Korea
A solid-state route was used to prepare sodium titanium oxide (NTO, Na2Ti3O7) as a solar heat protecting material with an impressive solar reflectance (TSR = 94.3%) using a high refractive index rutile TiO2. The solar reflectance of the synthesized NTO was measured using UV-Vis-NIR spectrophotometer. Solar reflectance property of the synthesized compound depends on the calcination temperature. The solar reflectance property of the synthesized NTO powder was compared with commercial rutile TiO2. The compound synthesized at 900 ℃ for 24 hrs had remarkable solar reflectance 94.3% than that calcined below 900 ℃. Crystalline nature, structural property, morphology and optical properties of NTO powders were characterized and analyzed using XRD, FE-SEM, EDS and UV-Vis-NIR spectrophotometer. From the results, we guessed that NTO would be a suitable “solar heat protecting candidate” for energy-saving applications in coating industries.
Keywords: Sodium titanate, Solid-state reaction, Titanium dioxide, Urban heat island effect, Solar heat reflectance, Cool roof material
In urban areas intensive urbanization and huge population growth play a major role on increasing environmental pollution, in these areas, the roofs design of the buildings has a major influence on the heat absorption of sunlight, which could finally result global warming [1].
The hot buildings also known as “Concrete Jungle” radiates heat and warms the air in the surrounding, such buildings in the vicinity combined effect and lead to a phenomenon known as ‘Urban Heat Island Effect’. In the surroundings, the amount of heat radiated varies depending on the type of coating materials, elevation and roof construction. By means of conduction, a significant amount of heat is also absorbed into the buildings. Increasing heat energy in such buildings, there is a need for capable energy in the form of cool air to keep interiors of the building reliable and tolerable for people to work and live in them [2].
For creating a cool community there is a need of lowering average surface temperature of the roof, therefore cooler roofs are very important in order to reduce the increasing demand for energy crisis for air conditioning. Most of the solar reflecting heat energy reduces the amount of energy absorbed by the building.
Solar radiations falling on an object meets three fates. It can be absorbed, reflected or transmitted. These all phenomenon can occur on their entire spectrum, it can absorb in ultraviolet (UV) range, reflect in visible region or may transmit at infra-red region [2, 3]. About 5% of the sun energy falls in the UV region that starts from 295 nm, because their shorter wavelengths this kind of energy is mostly responsible for breaking several kinds of bonds in organic and inorganic materials and also bound to cause sunburns. Aouut 50% of the sun energy falls in the visible region that starts from 400 to 700 nm. Usually, colored materials like green, yellow and red absorbs the visible light and this phenomenon is occurred because of light energy promotes electrons from one energy level to another which results the characteristic color of the material, while reflection of light around visible region are the characteristics properties of white materials. About 45% of the total solar energy consists of infrared red region and it starts from 700-2500 nm [4].
Usually heat have direct relation with infrared region and especially heat producing regions starts from 700-1100 nm range, therefore infrared reflective materials are the need of the day, and are increasingly used for coating of buildings because of their excellent property to enhance reflectivity in the IR region and to minimize heat producing issue in coating industry [4, 5]. Similarly, infrared reflective materials are very stable to high heat, keeps roof cooler, reduce “urban heat island effect” and still remains their color for longer time. The aim of solar reflective materials is to keeps objects cool and tolerable, which is normally obtained by using white coating. A selection of coatings can be added to the structure, and these coatings molecularly interact with infrared radiations to reflect or absorb the photons being emitted towards its surface. The surface chemistry dominates the interaction of infrared radiation on a surface of specified material [6].
For blocking the solar radiations there are two major ways: (a) reflection, which blocks the incoming heat waves, and (b) insulation, which prevents conduction and convection, and these phenomenon has been studied by a lot of researchers in the field of materials science, chemistry and engineering on their heat transfer behavior [5, 6], among them titanium with low cost, nontoxicity and more abundance has increased much attention for industrial applications which has been used normally to reflect solar radiations [7]. TiO2 is also an important coating candidate for improving the quality, durability, brightness and opacity of the materials [7, 8].
Currently various solar reflective materials have been researched, but the current need is to produce and develop cheaper, cooler and nontoxic materials for energy saving applications [9].
In recent years impressive solar reflective materials (with reflectance value of 90%-93.7%) having different shades are reported [10, 11].
Interestingly, “Sodium Titanate” Na2Ti3O7 has attracted more attention for its optical properties non-toxicity, low cost and stability [12], photocatalytic behaviour [13], as a low cost anode material [13] and nanosheets synthesis [14].
NTO accounts in an alkali-metal titanate family with a formula A2 Tin O2n + 1 (A = H, Li, Na and K while n = Ti starts from 3~8) which crystallizes in a monoclinic stuctures. Titanates with n = 3 or 4 consists of stable layers (Ti3O7)2- held together by sodium or potasium ions (Na+, K+) [15,16].
Trititanates i.e, (Na2Ti3O7) accounts in layered titanium-oxygen octahedra, in which the interlayer regions are occupied by sodium metals by a strong coulombic forces, and thus their crystal nature suggests the possibility of ionic conductions inside the layers, and also the possibility of ions exchangers. On the basis of their layered structures and ionic exchange behaviour various papers have been docummented [17-19]. Even though NTO has diverse applications in the field of chemistry, materials science, but to the best of our knowledge NTO has not been studied yet with such impressive solar reflective property.
According to our research work NTO calcined at different temperture is briefly discussed, their crystalline structure, composition, morphology and optical properties have been analyzed and compared with the commercial rutile TiO2. We therefore focused on improving the solar reflectance properties of NTO, and the influence of their calcination temperatures on their impressive solar reflectance. Thus, NTO would be an ideal candidate for protecting solar heat.
In our present study, the samples were prepared by performing two synthetic experimental steps. First, the precursor Na2Ti3O7 was prepared by using a previously reported conventional solid-state reaction method [20, 21]. A mixture of alkali metal carbonate Na2CO3 (purity 99.95%, Aldrich), and 2 types of commercial rutile TiO2 (<100 nm, Degussa; ≒1 mm, Inter-China Chem.) were used as raw materials. According to Eq. 1, the stoichiometric amounts of Na2CO3 and rutile TiO2 (100 nm or 1 mm) powders 1:3 were homogeneously mixed by using mortar and pestle and was calcined from 700 ~ 800 oC for 44 hrs or at 900 oC for 22 hrs with a heating rate of 5 degrees/minute in the presence of air. After calcination, the samples were reground and the same calcination temperature and time was applied once again.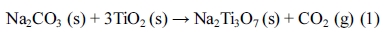
X-ray powder diffraction analysis (XRD, Rigaku Corp, DMAX 2500) was performed by using an internal radiation source Cu Kα (λ = 1.54056 Å), and the data were collected in the 2θ range of 5 ° to 70 ° with a scan speed of 5 degree/minute. Sodium titanate surface morphology were analyzed by using field-emission scanning electron microscopy (FE-SEM, JSM 6700F, JEOL Ltd.). Solar spectral reflectance of the samples was measured by a UV-Vis/NIR spectrophotometer (JASCO, V-570). While solar reflectance of the samples was measured at a wavelength range of 280-2500 nm. The following equation (Eq. 2) was used for calculating reflectance data of the samples [6].
Where R (%) means the solar reflectance in percent, Rλ indicates spectral reflectance, while Iλ indicates the solar spectral irradiance.
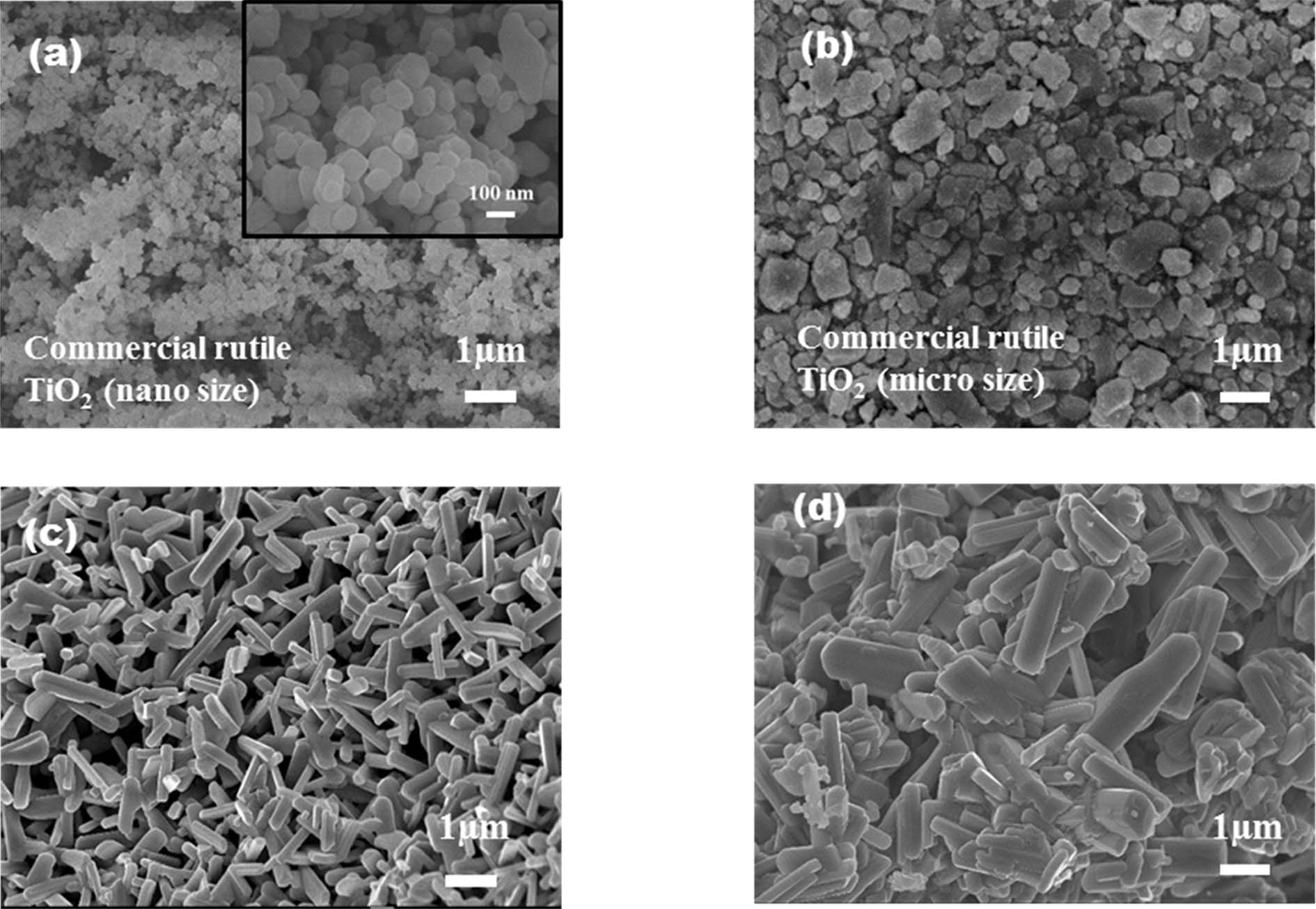
The surface morphology of the samples was characterized by using FE-SEM. Fig. 1(a) and 1b show the precursors rutile TiO2 with different particle sizes such as, 100 nm and 1 µm before heat treatment with sodium carbonate. After calcination a uniform flat-rod like morphology is shown by NTO that treated at 800 ℃ for 44 hrs by using 100 nm rutile TiO2, but different morphology with interpenetrating aggregates has shown by the product that calcined at 800 ℃ for 44 hrs by using 1 µm rutile TiO2, this phenomenon may be due the bigger particle size of TiO2 that has not been homogenously mixed with sodium carbonate during grinding. The results are given in Figs. 1(c) and 1(d).
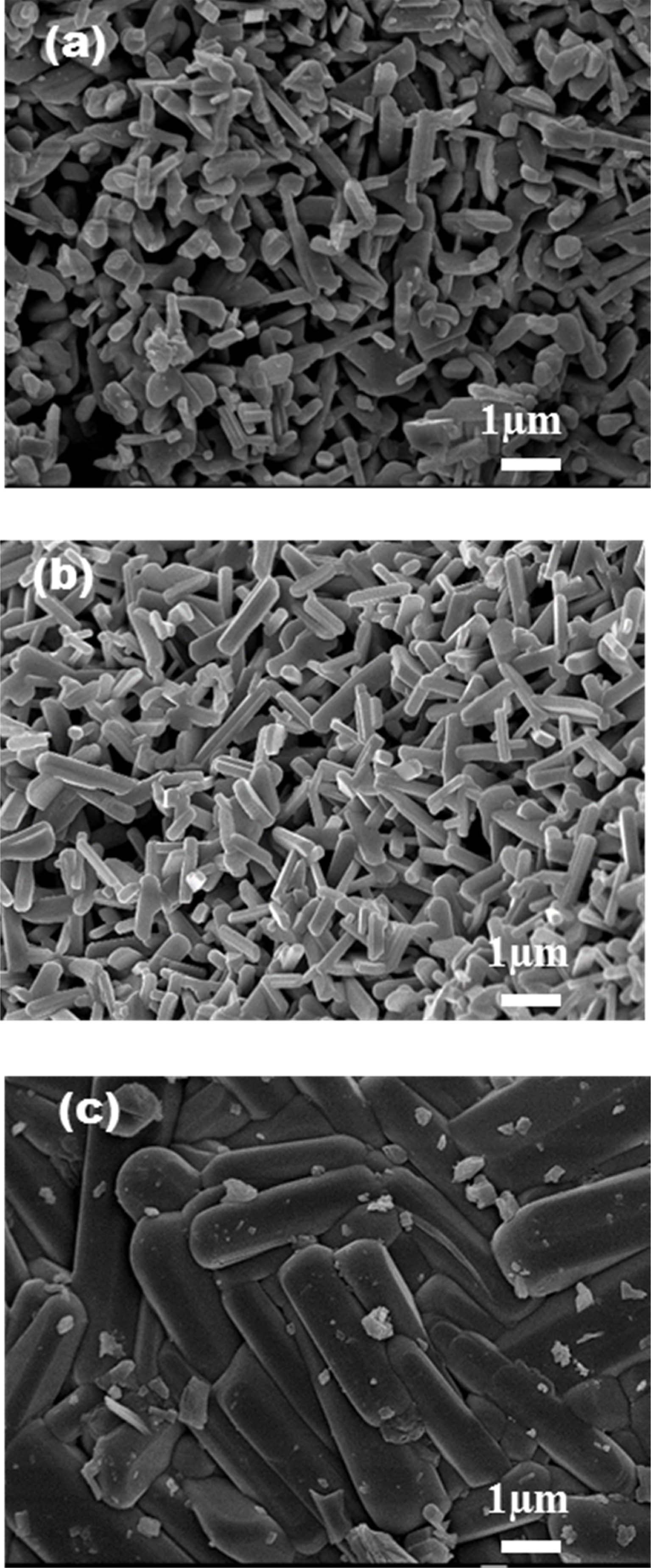
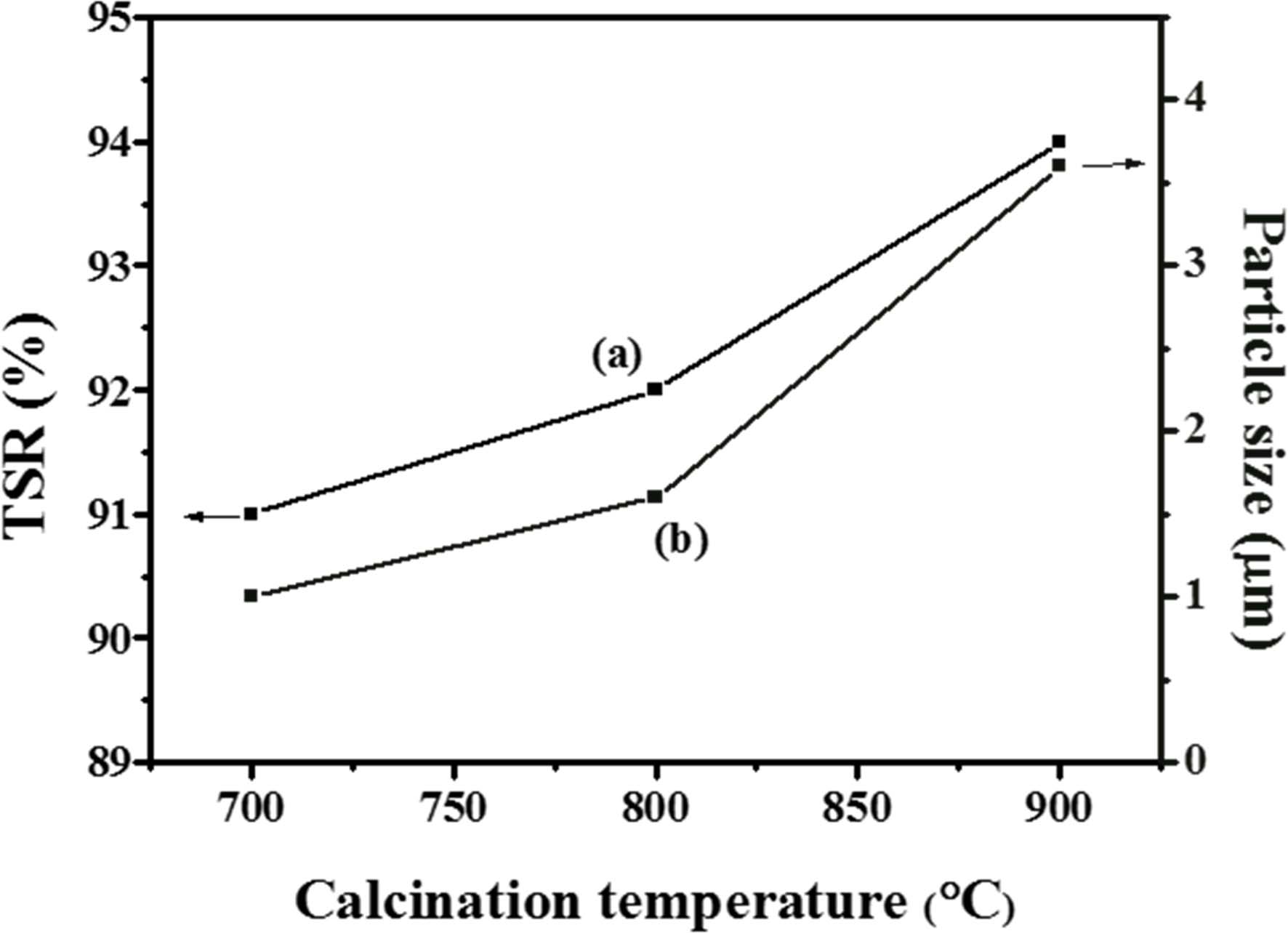
On the basis of uniform morphology of NTO, 100 nm rutile TiO2 was further calcined with Na2CO3 at 700, 800, and 900 ℃, respectively. For particle size measurement, at least 20 particles were averaged by using Mac-View Ver. 4 (MOUNTECH Co., Ltd.) software. About 1 µm particles was obtained for the sample that calcined at 700 ℃ for 44 hrs, about a length of 1.6 µm particles were obtained for the samples that calcined at 800 ℃ for 44 hrs, furthermore, the presence of smaller particles get disappears by using high calcination treatment, similarly 3.6 µm particles size with uniform morphology was obtained at 900 ℃ for 24 hrs. Fig. 2(a-c) indicate the FE-SEM images of the samples calcined at different temperatures.
|
Fig. 1 FE-SEM images of (a) 100nm and (b) 1 μm rutile TiO2 before calcination, (c) NTO sample calcined at 800 ℃ by using Na2CO3 and 100 nm TiO2 and (d) NTO sample calcined at 800 ℃ by using Na2CO3 and 1 µm TiO2. The inset in Fig. 1(a) shows rutile TiO2 with particle size of 100 nm. |
|
Fig. 2 FE-SEM images of NTO calcined at (a)700 ℃, (b) 800 ℃ and (c) 900 ℃ by using 100 nm rutile TiO2. |
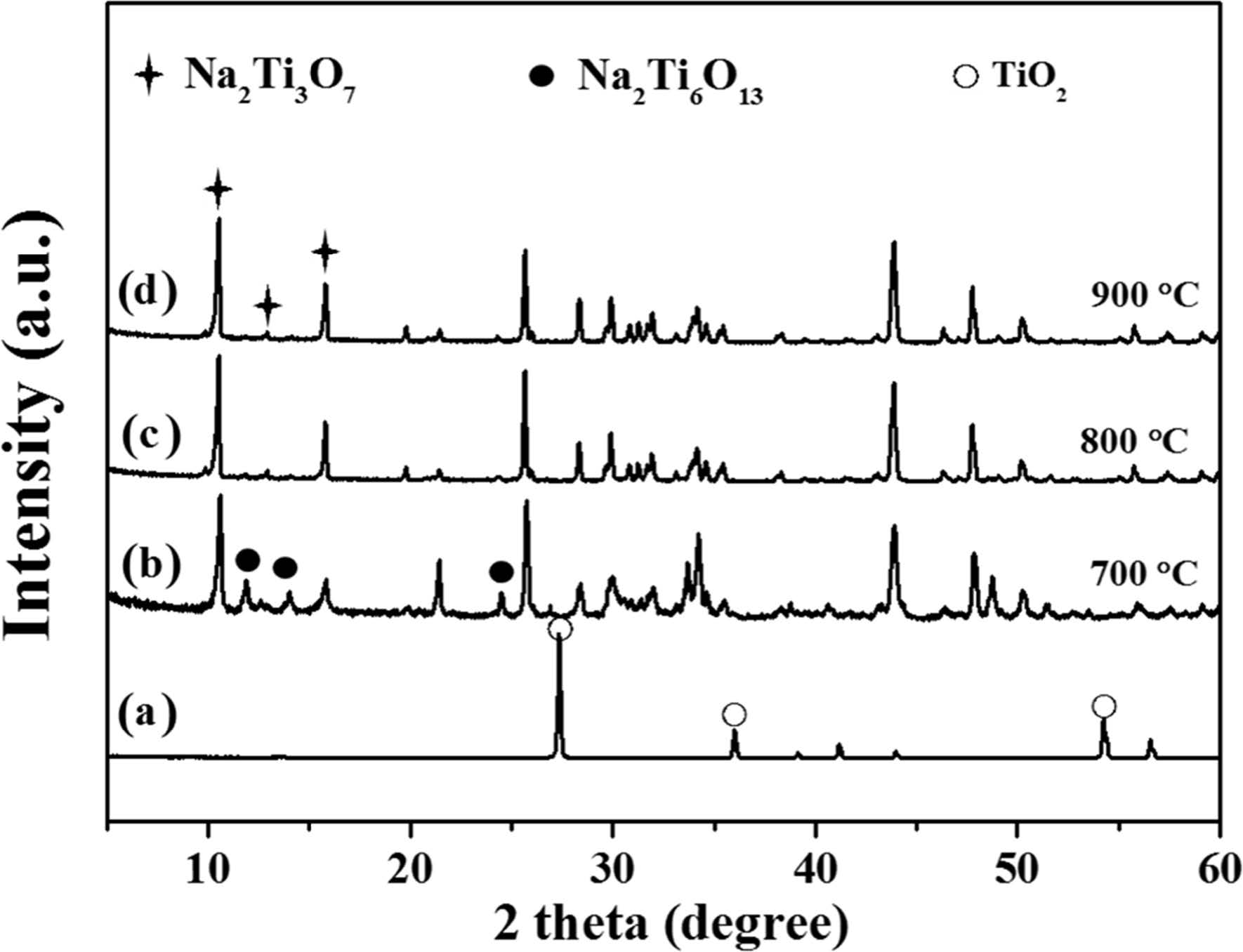
Depending on the different calcination treatment such as 700, 800, and 900 ℃, NTO was prepared by stoichiometric amount of precursors and were identified by using JCPDS files (PDF # 31-1329 for Na2Ti3O7). According to the XRD analysis, the sample calcined below 800 ℃ is a mixture of different phases as compared to samples treated at high temperature. Fig. 3 indicates the XRD patterns of commercial rutile TiO2 (100 nm) and the samples calcined at different temperature by using Na2CO3 and TiO2. In Fig. 3(b), the sample calcined at 700 ℃ for 44 hrs was obtained with various admixture phases in particular, Na2Ti3O7, Na2Ti6O13, Na2Ti4O9 and TiO2. The sample calcined at 800 ℃ for 44 hrs was appeared the major peak of Na2Ti3O7 and the minor peak of Na2Ti6O13 as shown in Fig. 3(c), but even at higher calcination temperature 900 ℃ in Fig. 3(d), the pure phase Na2Ti3O7 only appeared suggesting that higher calcination temperatures are necessary for reaction completion [20]. Therefore, a calcination temperature at 900 ℃ for 24 hrs has been chosen for a well-defined sodium titanate. It may be noted that the longer time of synthesis for the targeted “sodium titanate” (Na2Ti3O7) below 900 ℃ for 44 hrs does not lead to produce [22, 23]. The pure phase for the targeted “sodium titanate” could also be obtained by the use of rutile ‘titanium dioxide” (100 nm). Different phases detected by using XRD analysis for the calcined samples at various temperatures and times are summarized and given in Table 1.
|
Fig. 3 XRD patterns of (a) rutile TiO2, (b), (c) and (d) of NTO powders calcined at different temperature. |
|
Table 1 Experimental condition and phase analysis of NTO calcined at different temperatures. |
The infrared reflectance and total solar reflectance (IR and TSR) of the NTO powder was measured in the range of 280-2500 nm by using UV-Vis/NIR spectrophotometer. Especially IR region represents heat producing sites that starts from 700-1100 nm range. While TSR is the amount of measured reflected solar energy from a material surface, mathematically TSR is expressed as the integration of reflectance in percent times solar irradiance divided by the integral of the solar irradiance (280-2500 nm). Therefore, IR reflective materials are very important for coating applications, because they reflect heat waves and minimizes the sun’s heating energy absorbed by the buildings.
Usually white coating materials exhibit a TSR greater than 75% as compared to other colors [4, 5]. Similarly, NTO as a white powder also showed a remarkable solar reflectance as compared to commercial rutile TiO2.
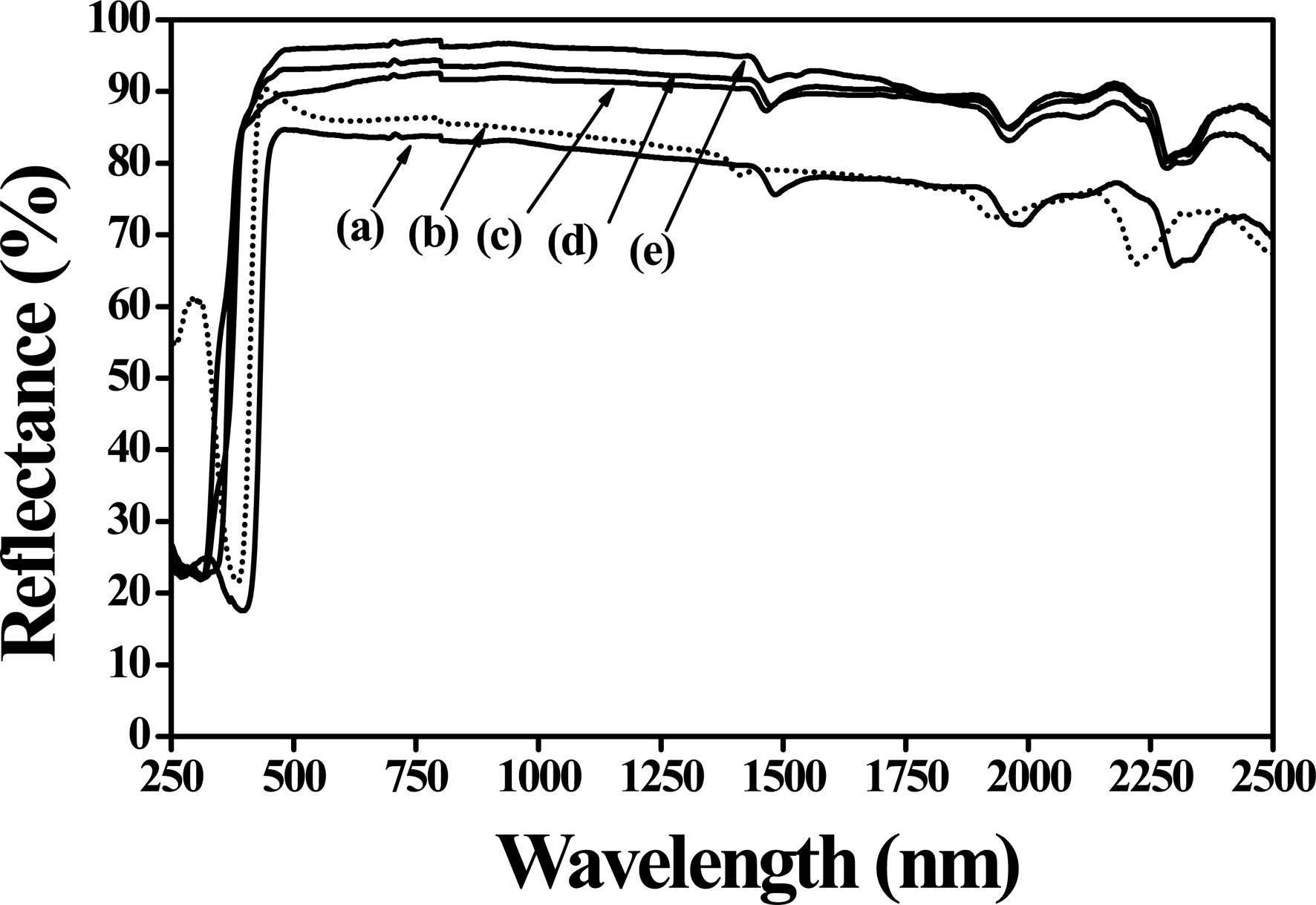
Fig. 4 displays the UV-Vis-NIR reflection spectra of five samples, which are commercial rutile titanium dioxide, Na2CO3/TiO2 (1:3 molar ratio) mixture before calcination, and three NTO powders calcined under 700, 800, and 900 ℃. As shown in Fig. 4(e), a higher solar reflectance of 94% shown by the sample that calcined at 900 ℃ for 24 hrs. Based on different calcination temperature NTO powder showed different solar reflectance, from lower calcination temperature to higher the solar reflectance values tends to higher. However, commercial rutile titanium dioxide and Na2CO3/TiO2 mixture have a lower total solar reflectance (TSR) of 72% and 82%, respectively. Generally high refractive index materials significantly tend to higher solar reflectance, this phenomenon may be due to the high refractive index value of rutile titanium 2.6 used in the compound or may be high whiteness of the NTO color [24]. However, no absorption was accounted in the visible region, this is because, in case of energy absorption the phenomenon is only happened when energy promotes electrons from one energy level to another, so this means there is no electronic excitation for absorbing energy from 400-700 nm [4, 5].
Fig. 5 indicates the solar reflectance of NTO in % and their comparison with their particle size based on Fig. 4. TSR of NTO calcined at different temperature and their comparison with commercial rutile TiO2 (100nm) and Na2CO3/TiO2 mixture are summarized in Table 2.
|
Fig. 4 UV-Vis-NIR reflectance spectra of (a) commercial rutileTiO2, (b) TiO2/Na2CO3 mixture (dotted line), while NTO samples calcined at (c) 700, (d) 800, and (e) 900 ℃. |
|
Fig. 5 Effect of calcination temperature and particle size on the total solar reflectance (TSR) of NTO. Line (a) means total solar reflectance in % and (b) means particle size in µm. |
|
Table 2 Solar reflectance properties of NTO samples calcined at different temperatures, commercial rutile TiO2, and mixture calculated based on Fig. 4. |

The color characteristics of the calcined NTO powder was investigated in 10% HNO3, HCl, and NaOH. A pre-weighted amount of NTO powder was mixed with acids and base in a 200 ml of a beaker and kept under a constant magnetic stirring for 2 hrs at room temperature. Furthermore, the resultant samples were vacuum-filtered, washed and dried under 60 ℃ for 24 hrs. The CIE L* a* and b* color scales was used for color measurement (CIE-LAB 1976 color scales). Where L* denotes the lightness, a* and b* denotes green/red and blue /yellow axis respectively [24]. The chemically treated samples color coordinates were compared with untreated NTO powder calcined at 900 ℃ and commercial rutile TiO2 (100 nm) are tabulated in Table 3. The L* a* and b* color coordinate of untreated NTO powder compared with acids and base treated samples was measured, the samples treated by NaOH and HCl show a negligible color change as compared to NTO, but in case of HNO3 treated samples, a little color variation has been occurred as compared to the original NTO.
In this work, Na2Ti3O7 (NTO) powder having an impressive infrared reflective and a total solar reflectance (TSR) of 94% was calcined by using simple solid state reaction. The solar reflective property of the synthesized NTO powder was measured in the range of 280-2500 nm by using UV-Vis-NIR spectrophotometer. It has been concluded that increasing calcination temperature from 700 ℃ to 900 ℃, have directly impacted on solar reflectance. The acid and base treated samples show better color stability, but a little color variation was shown in case of HNO3 treated sample. The TSR values (91~94%) of the synthesized NTO powder was compared to commercial rutile TiO2 (TSR = 72). The results show that the calcined NTO powder confirms their application as “cool material”. Furthermore, these results conclude that NTO would be a suitable cooler and cheaper candidate with high solar reflectance property for protecting heat and energy saving applications.
This work was financially supported by the Korean Evaluation Institute of Industrial Technology (KIET) (No. 10077449), funded by the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea.
- 1. U. Berardi and M. Naldi, Energy and Build. 144 (2017) 262-275.
-

- 2. V.C. Malshe, A. K. Bendiganavale, Recent Pat. Chem. Eng. 1 (2008) 67-79.
-

- 3. S. Bretz, H. Akbari, and A. Rosenfeld, Atmos. Environ. 32 [1] (1998) 95-101.
-

- 4. A. Libbra, L. Tarozzi, A. Muscio, and M.A. Corticelli, Opt. Laser Tech. 43[2] (2011) 394-400.
-

- 5. M. Sheikholeslami, D.D. Ganji, and R. Moradi, Chem. Eng. Sci. 173 (2017) 326-336.
-

- 6. H.J. Kim, H.J. Lee, and D.S. Kim, Mater. Des. 150 (2018) 188-192.
-

- 7. G.K. Dalapati, S.M. Panah, S.T. Chua, M. Sharma, T.I. Wong, H.R. Tan, and D. Chi, Sci. Rep. 6 (2016) 20182.
-

- 8. S.-Z. Zhang, W. Shi, Y. Song, J. Qu, J. Qin, J. Zhang, T. Li, Y. H. Zhang, and R. Zhang, J. Energy. Build. 63 (2013) 49-58.
-

- 9. R. Levinson, P. Berdahl, and H. Akbari, Sol. Energy Mater. Sol. Cells 89 (2005) 351-389.
-

- 10. D. Schildhammer, G. Fuhrmann, L. Petsching, N. Weinberger, H. Schottenberger, and H. Huppertz, Dyes. Pigm. 138 (2017) 90-99.
-

- 11. L.S. Kumari, P.P. Rao, A.N.P. Radhakrishnan, V. James, S. Sameera, and P. Koshy, Sol. Energy Mater. Sol. Cells. 112 (2013) 134-143.
-

- 12. A. Adailton, A.F. Fabio, L.R. Silvab, A. Righib, M.B. da Silvaa, B.P. Silvaa, E.W.S. Caetanoc, and V.N. Freirea, J. Solid. State Chem. 250 (2017) 68-74.
-

- 13. Y.C. Pu, Y.C. Chen, and Y.J. Hsu, App. Catal. B. Environ. 97 (2010) 389-397.
-

- 14. M. Zarrabeitia, E.C. Martínez, J.M.L Opez, D. Amo, A.E. Barrio, M.A. Munoz, M. arquez, T. Rojo, and M.C. Cabanas, J. Power Sources 324 (2016) 378-387.
-

- 15. Y. An, D. Wang, and C. Wu, Physica E. 60 (2014) 210-213.
-

- 16. T.-P. Feist, S.J. Mocarski, P.K. Davies, A.J. Jacobson, and J.T. Lewandowski, Solid State Ion. 28 (1988) 1338-1343
-

- 17. H. Izawa, S. Kikkawa, and M. Koizumi, J. Solid State Chem. 60 (1985) 264-267.
-

- 18. H. Izawa, S. Kikkawa, and M. Koizumi, J. Solid State Chem. 69 (1987) 336-342.
-

- 19. S. Takayoshi, F. Izumi, and M. Watanable, Chem. Mater. 8 (1996) 777-782.
-

- 20. M. Dynarowska, J. Kotwinski, M. Leszczynska, M. Marzantowicz, and F. Krok, Solid. State Ion. 301 (2017) 35-42.
-

- 21. M.A. Tsiamtsouri, P.K. Allan, A.J. Pell, J.M. Stratford, G. Kim, R.N. Kerber, P.C.M.M. Magusin, D.A. Jefferson, and C.P. Grey, Chem. Mater. 30 (2018) 1505-1516.
-

- 22. A. Rudola, K. Saravanan, C.W. Mason, and P. Balaya, J. Mater. Chem. A1 (2013) 2653-2662.
-

- 23. R.S. Dubey, and B. Ganesan, Super lattice Microst. 111 (2017) 1099-1103.
-

- 24. P. Meenakshi, and M. Selvaraj, Sol. Energy Mater. Sol. Cells 174 (2018) 530-537.
-

 This Article
This Article
-
2019; 20(S1): 86-91
Published on Jul 20, 2019
- Received on Dec 9, 2018
- Revised on Jan 11, 2019
- Accepted on Jan 11, 2019
 Services
Services
- Abstract
introduction
experimental section
results and discussion
conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Dae-Sung Kim
-
Energy Environment Division, Korea Institute of Ceramic Engineering and Technology (KICET), 101, Soho-ro, Jinju-si, Gyeongsangnam-do 52851, Korea
Tel : +82-55-792-2453
Fax: +82-55-792-2579 - E-mail: dskim@kicet.re.kr






 Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.
Copyright 2019 International Orgranization for Ceramic Processing. All rights reserved.